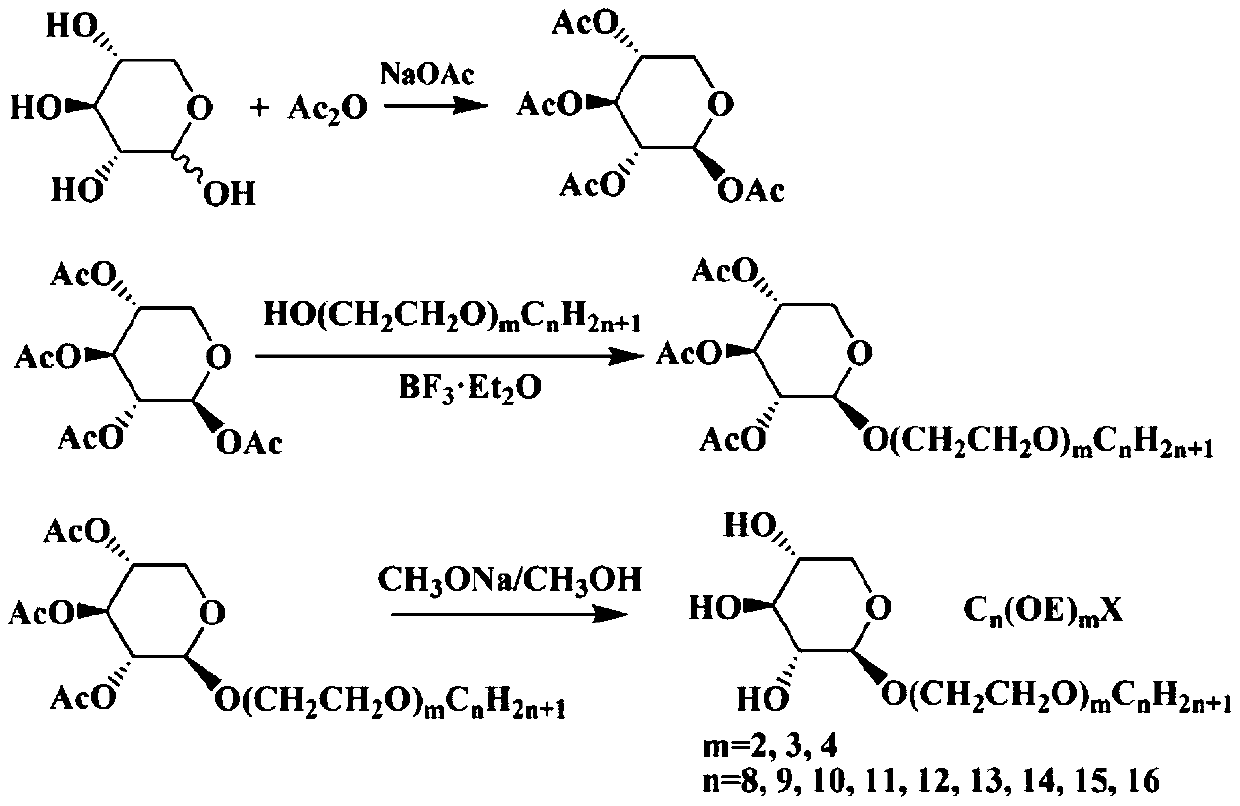
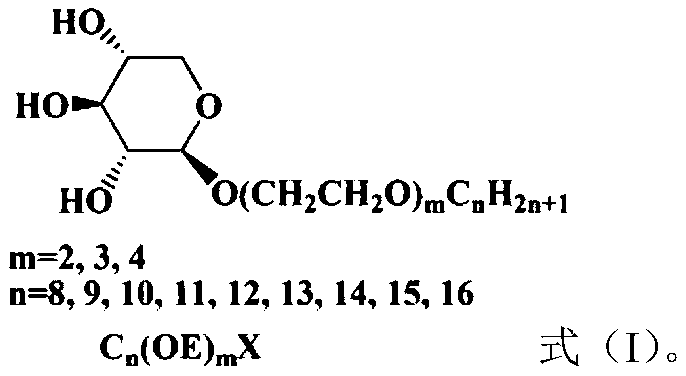1,2-trans alcohol ether xyloside surfactant and preparation method
A technology of xyloside and alcohol ether, which is applied in the field of fine chemical surfactants and can solve the problems of poor water solubility of alkyl glycosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0023] Add 0.33mol of dried D-xylose, 1.67mol of acetic anhydride and 83.26mmol of anhydrous sodium acetate into a 500mL three-necked flask, heat up to dissolve the solid slightly, then stop heating and continue stirring until the solid is completely dissolved. The reaction solution was clear and cooled to room temperature. Add 83.26mmol anhydrous sodium acetate again, heat to 110 ℃ reflux reaction again, TLC (developing agent: V 石油醚 :V 乙酸乙酯 = 1:1) Monitor the reaction to the end point. When it was slightly cold, the reaction solution was poured into ice water and stirred while it was hot, and a large amount of solids were precipitated immediately, filtered with suction, and the filter cake was washed several times with water, and dried to obtain 78.60 g of acetyl-protected D-xylose, with a yield of 74.8%. The compound was washed with aqueous methanol (V 甲醇 :V 水 =1:2) After recrystallization and purification, it was directly used in the reactions in subsequent examples.
Embodiment 1
[0025] Embodiment 1: Octyldipolyoxyethyl-β-D-xylopyranoside (C 8 (OE) 2 X)
[0026] (1) In the round-bottomed flask, add successively prepared 22.42mmol of acetyl-protected D-xylose, Molecular sieve-dried 50mL dichloromethane and 33.63mmol diethylene glycol monooctyl ether were stirred and dissolved, cooled to 0°C, 67.27mmol boron trifluoride diethyl ether was added dropwise, the temperature was naturally raised to room temperature and stirred for reaction, TLC (developing agent: V 石油醚 :V 乙酸乙酯 =2:1) Monitor the progress of the reaction, and the reaction is completed in 3h. The mixed solution was washed successively with saturated aqueous sodium bicarbonate solution and saturated saline solution, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated, and subjected to column chromatography (V 石油醚 :V 乙酸乙酯 =5:1) separation to obtain 7.06 g of triacetyloctyldipolyoxyethyl-β-D-xylopyranoside, with a yield of 66.1%.
[0027] (2) Add 14.82mmol of triac...
Embodiment 2
[0030] Embodiment 2: nonyldipolyoxyethyl-β-D-xylopyranoside (C 9 (OE) 2 X)
[0031] The method is similar to Example 1. (1) The dosage is specifically: 22.29mmol acetyl-protected D-xylose, Molecular sieve-dried 50mL dichloromethane, 33.44mmol diethylene glycol monononyl ether, 66.87mmol boron trifluoride ether; 3h to complete the reaction; through similar post-treatment, the ratio of the eluent separated by column chromatography for V 石油醚 :V 乙酸乙酯 =5:1, 7.41 g of triacetylnonyldipolyoxyethyl-β-D-xylopyranoside was obtained with a yield of 67.8%. (2) The dosage is specifically: 15.11 mmol of triacetylnonyldipolyoxyethyl-β-D-xylopyranoside, 40 mL of anhydrous methanol, and the reaction is completed in 2 hours. Through similar post-treatment, 4.81 g of nonyldipolyoxyethyl-β-D-xylopyranoside was obtained with a yield of 87.3%.
[0032] nonyldipolyoxyethyl-β-D-xylopyranoside 1 H NMR, mass spectrometry test data are as follows:
[0033] 1 H NMR (D 2 O)δ4.37(d,J 1,2 =7.7H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com