Rabies virus antibody test paper, preparation method thereof and detection method thereof
A rabies virus and antibody detection technology, applied in the field of rabies virus antibody detection test strips and their preparation, can solve problems such as false positives, and achieve the effects of avoiding false positives, practical and effective detection methods, and improving accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
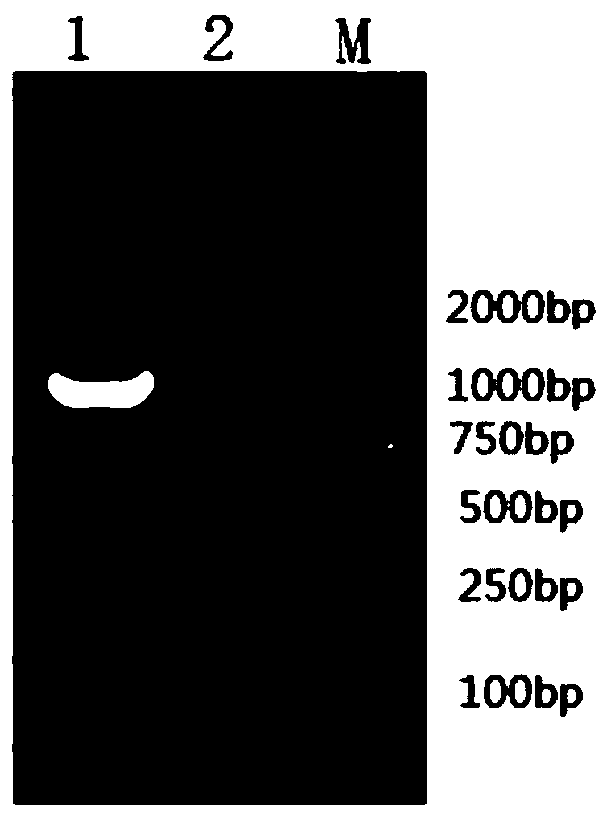
[0036] Example 1 Preparation and identification of rabies virus-like particles
[0037] (1) Material
[0038] The donor plasmid pFastBac1 was purchased from Invitrogen Company, and the strain E.coli DH10Bac, insect cell Sf9, and rabies virus were provided by Changchun Zhuoyi Biological Co., Ltd.
[0039] Rabbit anti-RABV MP serum was made by Changchun Sino Biotechnology Co., Ltd.; Phusion ultra-fidelity DNA polymerase and T4 DNA ligase were purchased from NEB Company; restriction endonuclease and protein marker were purchased from Thermo Company; DH5α competent cells , DNA Molecular Marker, and BacPAK Baculovirus Rapid Titer Detection Kit were purchased from Takara Company; plasmid mini-extraction kits, DNA gel recovery kits, and genome extraction kits were purchased from Axygen Company; cell transfection reagent CellfectionII Reagent was purchased from Invitrogen Company; fetal bovine serum is a product of GIBCO Company.
[0040] (2) Method
[0041] (a) Extraction of viral...
Embodiment 2
[0064] Preparation and identification of embodiment 2 rabies virus G protein monoclonal antibody
[0065] (1) Select 5 female BALB / c mice aged 6-8 weeks, inject the aforementioned purified rabies virus G protein virus-like particles and the same amount of Freund's complete adjuvant emulsion into the mice, and inject 200 μl subcutaneously into each mouse . Two weeks later, the second immunization was carried out, and the mice were injected with rabies virus G protein virus-like particles and an equal amount of Freund's incomplete adjuvant emulsion, and the injection volume and method were the same as the first immunization. The third immunization was carried out two weeks after the second immunization, and the immunization method and virus injection volume were the same as the second immunization. Two weeks after the third immunization, when the serum ELISA titer reached 1:10000 or more after the tail-cut blood collection, the splenocytes and myeloma cells were removed at 6×10...
Embodiment 3
[0067] The preparation of embodiment 3 rabies virus antibody quantitative detection kit
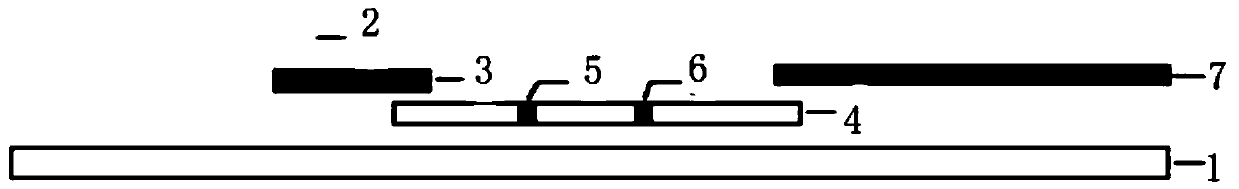
[0068] (1) Preparation of sample pad
[0069] The purified rabies virus-like particles prepared in Example 1 were diluted to 1.0 mg / ml with 20 mM sodium tetraborate solution, containing 2% Tween-20, and sprayed on the glass cellulose membrane as a sample pad.
[0070] (2) Preparation of gold standard pad
[0071] With 0.2mol / L K 2 CO 3 After adjusting the pH value of the 25nm colloidal gold solution to 8.4, add the rabies virus-like particle monoclonal antibody to the colloidal gold solution under rapid stirring according to the protein concentration of 50 μg / ml of the qualified rabies virus-like particle monoclonal antibody after purification And continue to stir for 30 minutes; add 10% BSA to a final concentration of 1%, stir for 30 minutes, centrifuge at 10,000r / min for 30 minutes, carefully suck off the supernatant, and the precipitate is the initially purified gold-labeled rabies ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein concentration | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com