Synthesis method of p-trifluoromethyl benzonitrile compound
A technique of trifluoromethylbenzonitrile and a synthetic method, which is applied in the field of pesticide chemistry, can solve problems such as long reaction time, high industrialization cost, and difficult marketization, and achieve selectivity and conversion rate improvement, simplification of reaction steps, solid Simple Effects of Waste Ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
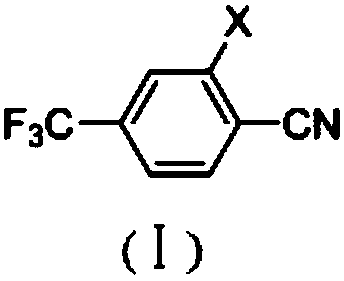
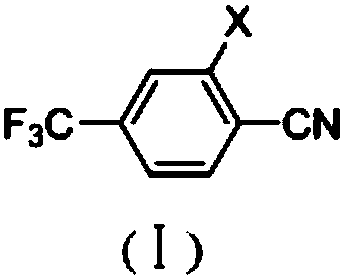
[0048]Under argon protection, 227.8g (1mol) of 3-nitro-4-chlorobenzotrifluoride, 108.6g (1.2mol) of cuprous cyanide, 41.6g (0.4mol) of sodium bromide, 400.0 g of dimethyl sulfoxide was heated to 150° C. under stirring, and the reaction was kept for 5 hours. According to GC analysis, the conversion rate is 97.2%, and the selectivity is 96.27%. DMSO was removed under reduced pressure, 500g of toluene was added, 81g of inorganic salt was reclaimed by filtration, the mother liquor was collected, toluene was reclaimed under reduced pressure, and rectification under reduced pressure gave 200.6g of 2-nitro-4-trifluoromethylbenzonitrile, yield 92.1%, purity 99.2%.
[0049] The mass spectral data (EI) of 2-nitro-4-trifluoromethylbenzonitrile is: 216 (M + )197(M-F)186(M-NO)170(M-NO 2 , base); 1 H NMR (d 6 -DMSO, 500 MHz): δ 8.41 (m, 2H), 8.65 (s, 1H).
Embodiment 2
[0051] Under argon protection, 227.8g (1mol) of 3-nitro-4-chlorobenzotrifluoride, 108.6g (1.2mol) of cuprous cyanide, 41.6g (0.4mol) of sodium bromide, 400.0 g of N,N-dimethylformamide was heated up to 140° C. under stirring, and kept for 7 hours for reaction. GC detected that the conversion rate was 96.1%, and the selectivity was 93.3%. DMF was removed under reduced pressure, 500g of toluene was added, the inorganic salt was recovered by filtration, the mother liquor was collected, the toluene was recovered under reduced pressure, and rectification under reduced pressure gave 194.2 g of 2-nitro-4-trifluoromethylbenzonitrile with a yield of 88.5 %, purity 98.4%.
Embodiment 3
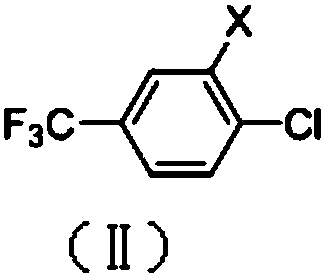
[0053] Under the protection of argon, 217.2g (1mol) of 3,4-dichlorobenzotrifluoride, 108.6g (1.2mol) of cuprous cyanide, 41.6g (0.4mol) of sodium bromide, and 400.0 g, the temperature was raised to 160° C. under stirring, and the temperature was kept for 7 hours. GC detected that the conversion rate was 84.2%, and the selectivity was 93.1%. Removed DMSO under reduced pressure, added 500g toluene, recovered inorganic salts by filtration, collected mother liquor, recovered toluene under reduced pressure, and rectified under reduced pressure to obtain 155.8 g of 2-chloro-4-trifluoromethylbenzonitrile with a yield of 781.3% , 98.8% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com