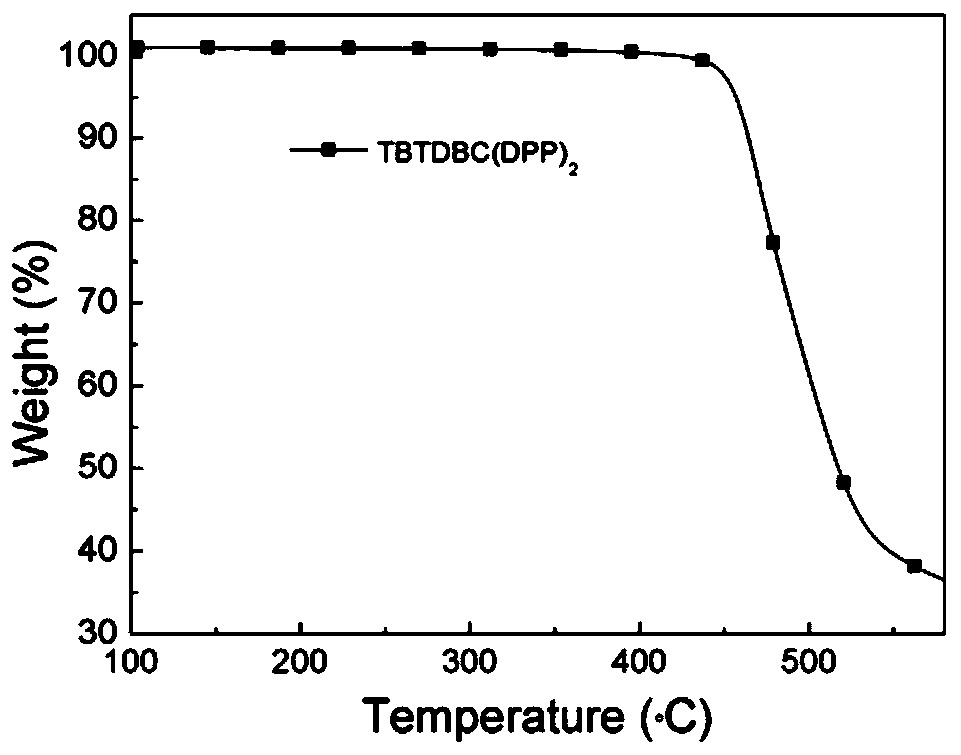
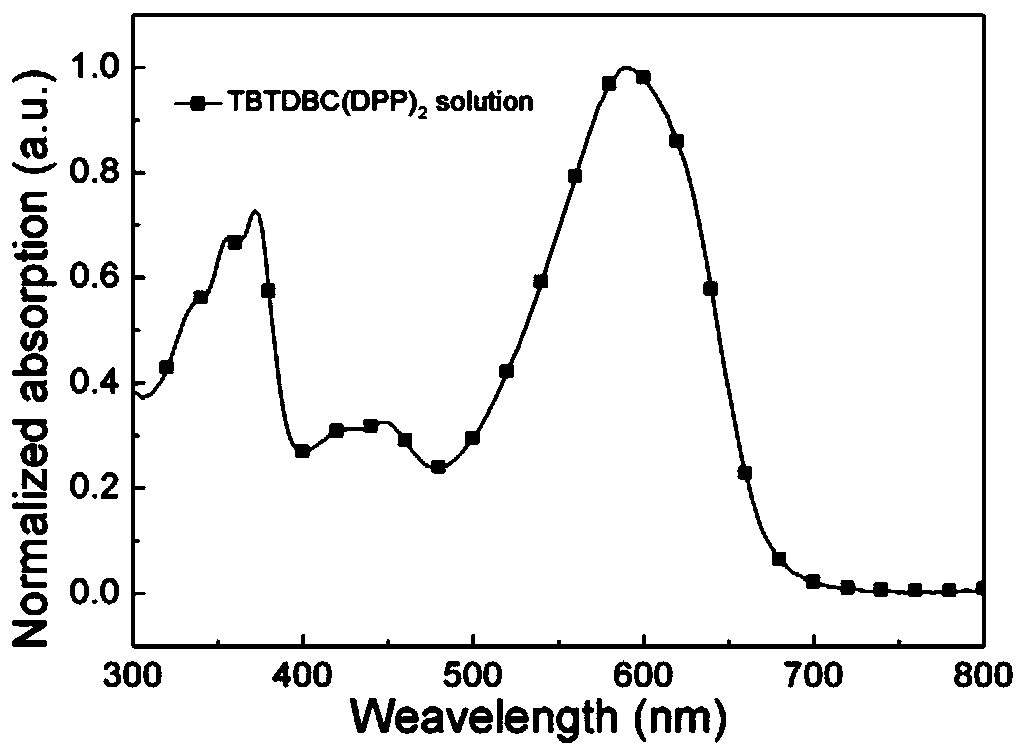
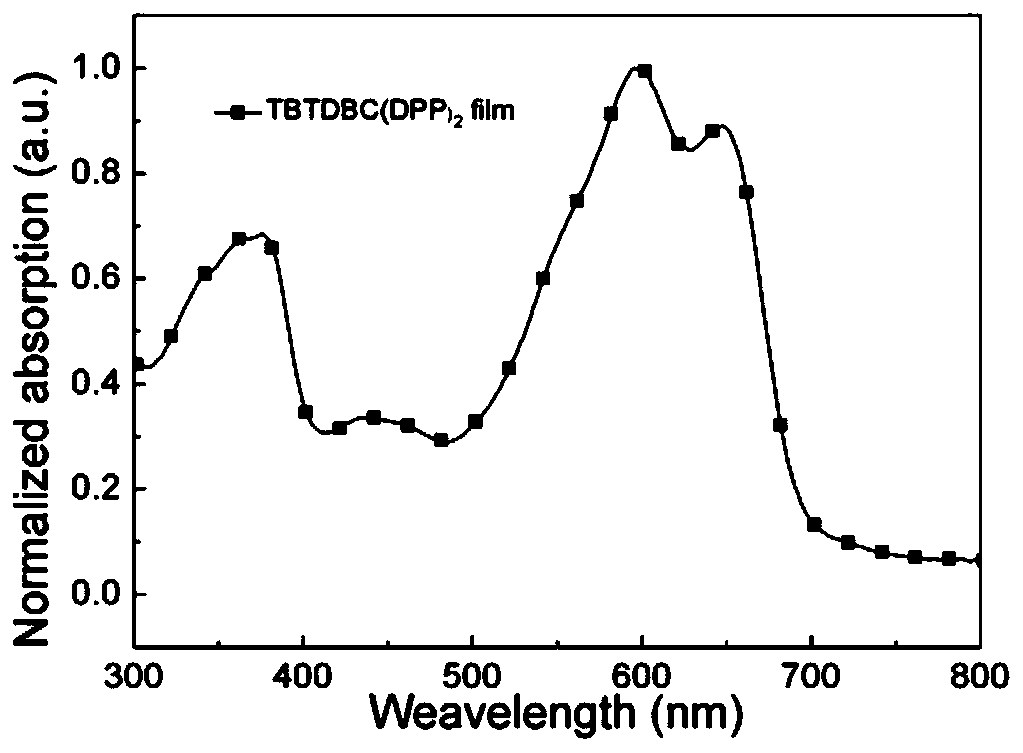
D (A-Ar) 2 type organic photoelectric compound based on carbazole eleven-membered fused ring planar nucleus and a preparation method and application thereof
A compound, carbazole technology, applied in the field of organic optoelectronics, can solve the problems of low carrier mobility, narrow absorption spectrum, low efficiency, etc., and achieve the effects of wide ultraviolet absorption, high batch repeatability, and molecular weight determination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] D(A-Ar) based on 11-membered fused ring core of carbazole 2 The synthetic route of the intermediates (DPP-Cz, Br-DPP-Cz, DPP-Th, Br-DPP-Th, DPP-ST, Br-DPP-ST) of the linear small molecules of organic optoelectronic compounds is shown in the figure below.
[0089]
[0090] 1.1, 2,5-bis-(2-hexyldecyl)-3-(5-(9-octylcarbazol-3-yl)thiophen-2-yl)-6-(thiophen-2-yl) Synthesis of pyrrolo[3,4-c]pyrrole-1,4-dione (DPP-Cz)
[0091] Add 2,5-bis(2-hexyldecyl)-3-(5-bromothiophen-2-yl)-6-(thiophen-2-yl)pyrrolo[3,4 -c] pyrrole-1,4-dione (Br-DPP) (0.50 g, 0.60 mmol), 30 mL of toluene, tetrakis (triphenylphosphine) palladium (Pd (PP 3 ) 4 , 35mg, 0.03mmol), 9-octyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-carbazole (267mg, 0.66 mmol), potassium carbonate solution (2M, 3mL) and Aliquant 336 (4 drops). Reflux for 24 hours, cool down, extract with 3×25mL chloroform, combine the organic phases, wash with 3×40mL saturated brine, dry the organic phase with anhydrous magnesium sul...
Embodiment 2
[0103] Based on D(A-Ar) 2 TBTDBC-[Sn(CH 3 ) 3 ] 2 The synthetic route of is shown in the figure below.
[0104]
[0105] 2.1 Synthesis of 2,7-dibromo-3,6-diiodo-9-(2-octyldodecyl)carbazole (M1)
[0106] In a 100mL three-necked flask, add 2,7-dibromo-carbazole (3g, 4.97mmol) and 50mL acetic acid successively, pass nitrogen for deoxygenation replacement three times, stir magnetically, dissolve the reactants at 80°C, and quickly weigh potassium iodide (2.22 g, 13.41mmol) and potassium periodate (1.43g, 6.71mmol), and sequentially added to the reaction system, reflux for 12h. After the reaction was complete, it was cooled to room temperature, and the mixture was quenched by pouring into 100 mL of water, and extracted with chloroform (100 mL×3) and washed with saturated sodium sulfite solution (100 mL×3). Combine the organic phases and wash with anhydrous MgSO 4 Dry overnight, filter, and spin off the solvent under reduced pressure to give a gray crude product. The concen...
Embodiment 3
[0116] D(A-Ar) based on 11-membered fused ring core of carbazole 2 Type target compound TBTDBC(DPP) 2 , TBTDBC (DPP-Cz) 2 , TBTDBC (DPP-Th) 2 and TBTDBC (DPP-ST) 2 The synthetic route of is shown in the figure below.
[0117]
[0118] where Ar is:
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| optical band gap | aaaaa | aaaaa |
| optical band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com