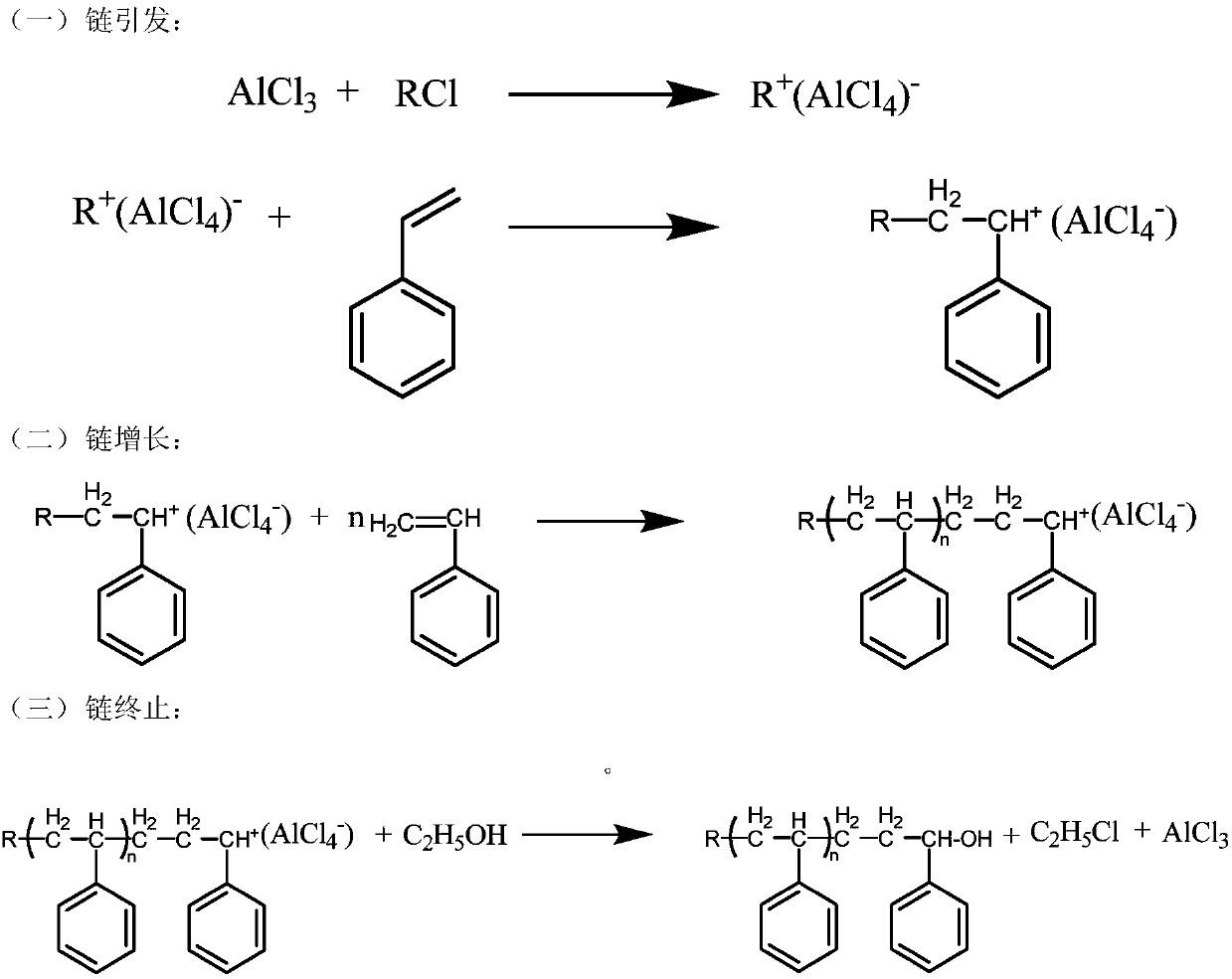
Method for preparation of organic porous material based on cationic polymerization reaction
A porous material and organic technology, applied in the field of rapid preparation of organic porous materials based on cationic polymerization, can solve the problems of harsh reaction conditions and long reaction time, and achieve the effects of short reaction time, low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047]The solvent used in the preparation method of the organic porous polymer of the present invention is a halogenated solvent, preferably one or more of methyl chloride, methylene chloride, ethylene dichloride, chloroform or carbon tetrachloride.
[0048] The applicant of the present invention found in experiments that Lewis acid or protonic acid can be used as a catalyst to catalyze monomer polymerization, wherein anhydrous aluminum trichloride, anhydrous ferric chloride, anhydrous tin tetrachloride, anhydrous chlorinated Both zinc and concentrated sulfuric acid can catalyze the reaction polymerization. Among them, anhydrous ferric chloride and anhydrous aluminum trichloride have the highest catalytic activity and the fastest reaction rate, while the catalytic activity of zinc chloride is low, and dilute sulfuric acid cannot catalyze the reaction because the water content in dilute sulfuric acid is too high , water will quench the cationic active center.
[0049] The dosa...
Embodiment 1
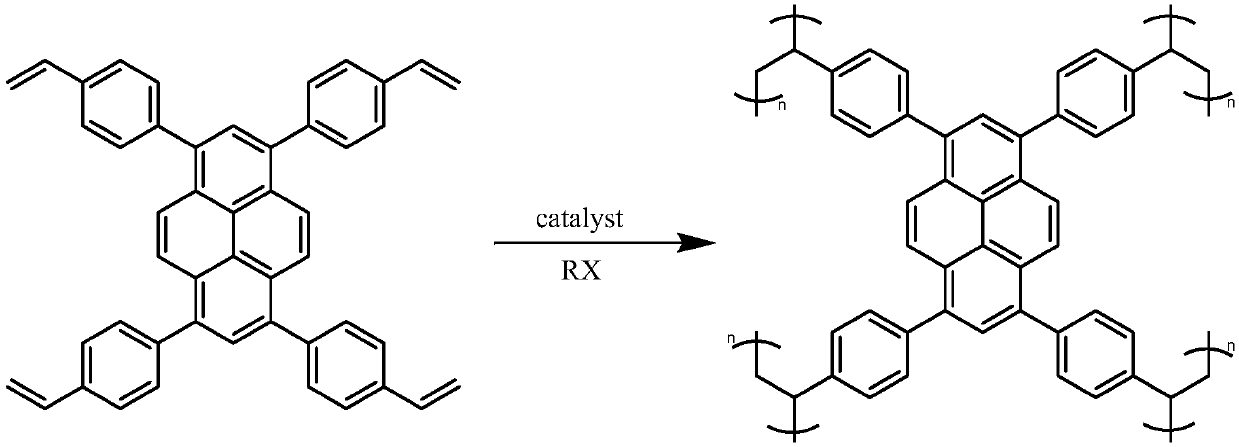
[0057] Take 0.122g of 1,3,6,8-tetrakis(4-vinylphenyl)pyrene (TVP), add 15ml of 1,2-dichloroethane (DCE), and ultrasonically disperse evenly to obtain a green solution, stir, add 0.053g AlCl 3 , the color gradually became darker, dark green, and finally black, with bluish light on the edge of the liquid surface. After 36 hours of reaction, a large amount of ethanol was added to quench the reaction, and a large amount of light green precipitates were precipitated in the solution. Suction filter, wash with a large amount of ethanol several times, extract with ethanol for 24 hours, and dry in an oven at 60°C for 24 hours. A yellow solid was obtained in greater than 99% yield.
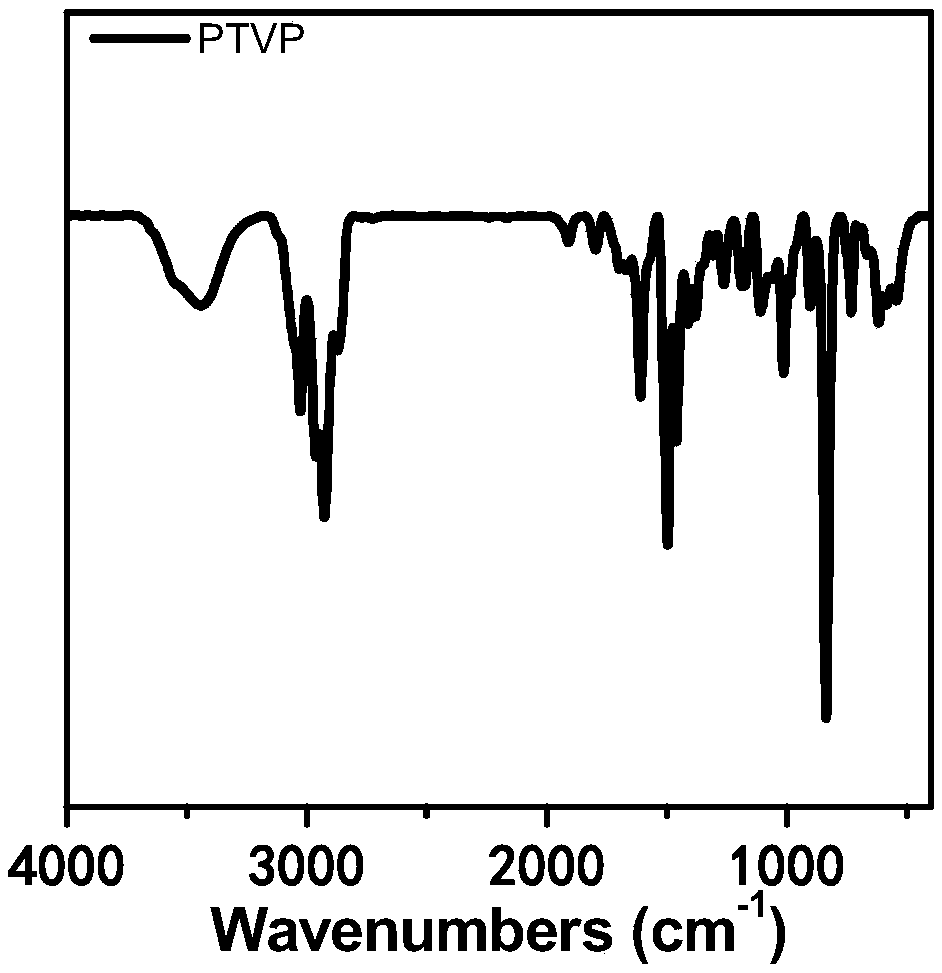
[0058] figure 2 It is a schematic diagram of the preparation reaction of this embodiment, and 1,3,6,8-tetrakis (4-vinylphenyl) pyrene has undergone a cross-linking polymerization reaction; image 3 , Figure 4 , Figure 5 with Image 6 Be respectively the infrared spectrum of embodiment 1 product, 1...
Embodiment 2
[0060] Take 0.122g of 1,3,6,8-tetrakis(4-vinylphenyl)pyrene (TVP), add 15ml of 1,2-dichloroethane (DCE), and ultrasonically disperse evenly to obtain a green solution, stir, add 0.065g FeCl 3 , the color gradually became darker, dark green, and finally black, with bluish light on the edge of the liquid surface. After 36 hours of reaction, a large amount of methanol was added to quench the reaction, and a large amount of light green precipitate was precipitated in the solution. Suction filter, wash with a large amount of methanol several times until the filtrate becomes colorless, extract with methanol for 24 hours, and dry in an oven at 60°C for 24 hours. A yellow-green solid was obtained in greater than 99% yield.
[0061] Figure 7 , Figure 8 , Figure 9 Be respectively the infrared spectrum of embodiment 2 product, nitrogen adsorption-desorption curve and pore size distribution curve, the BET of embodiment 2 product is 1549m 2 g -1 ,from Figure 8 with Figure 9 An...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com