Methotrexate transdermal local controlled release preparation and its preparation method and application
A technology of methotrexate and controlled-release preparations, which is applied in pharmaceutical formulations, antipyretics, anti-inflammatory agents, etc., can solve problems such as adverse reactions of patients, reduce the dosage of patients, achieve convenient storage and management of medicines, and improve light Stability, the effect of ensuring photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 mesoporous silica
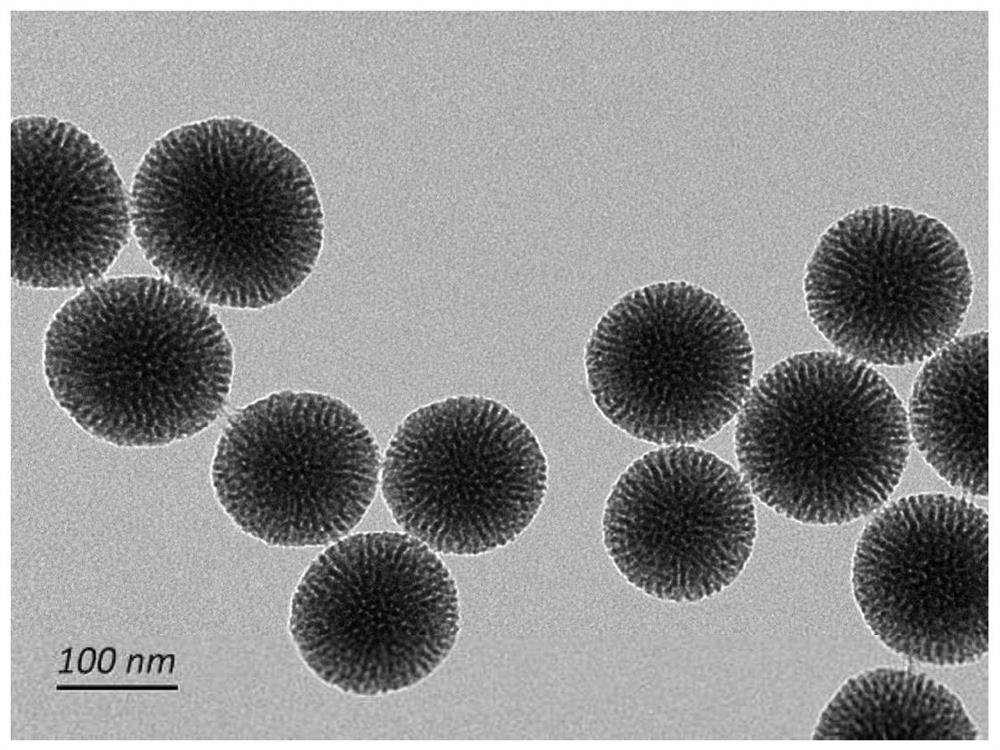
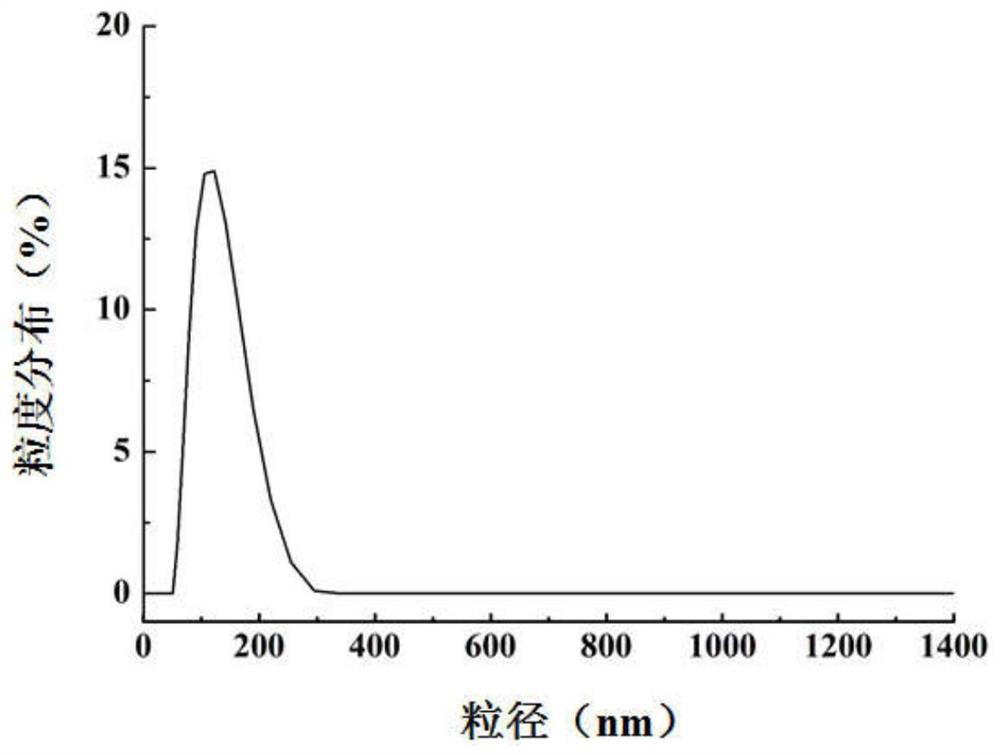
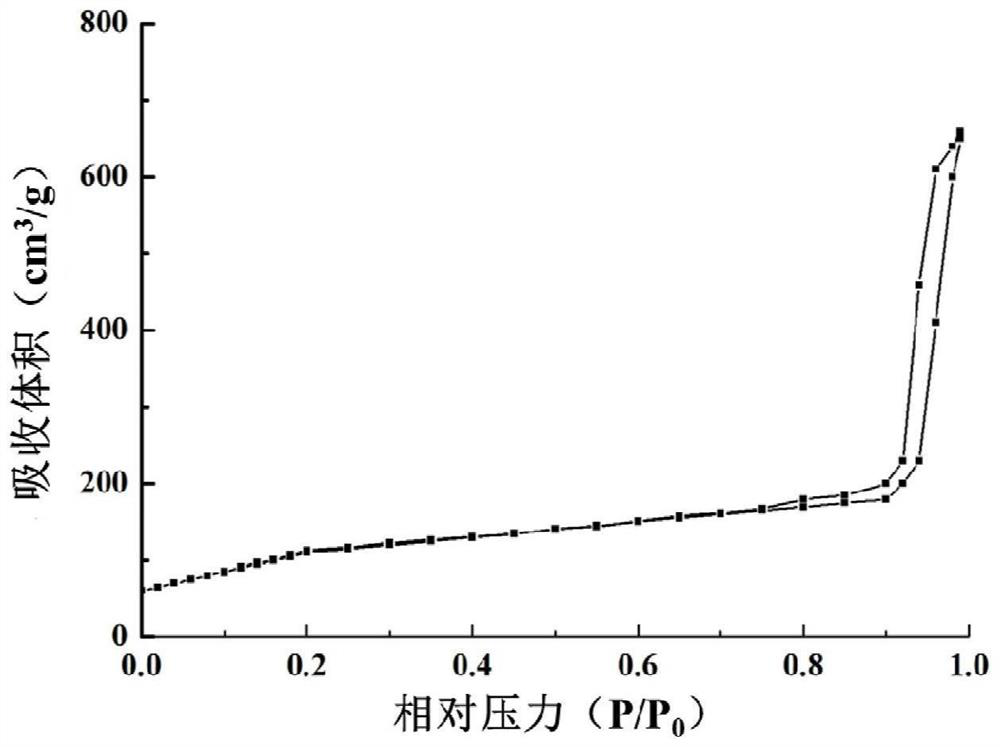
[0048]Add 24mL of cetyltrimethylammonium chloride (CTAC), 36mL of water, and 0.18g of triethanolamine (TEA) into a 100mL flask in sequence, and stir magnetically at a constant temperature of 80°C for 1h. The prepared mixture of 4 mL tetraethyl orthosilicate (TEOS) and 1 mL cycloethane was added dropwise and stirred slowly at 60° C. for 12 h. After the reaction is completed, wash with 0.6% ammonium nitrate ethanol solution three times in a 60° C. water bath, each time for 6 hours. After the reaction was completed, the ammonium nitrate was removed with absolute ethanol, and freeze-dried to obtain mesoporous silica. Characterization diagram of mesoporous silica Figure 1a and Figure 1b , Figure 1c , Figure 1d .
Embodiment 2
[0049] Embodiment 2. Methotrexate content assay method
[0050] (1) Chromatographic conditions
[0051] Use Shimadzu LC-6AD to detect methotrexate content, chromatographic column: Thermo ODS-2 C18 (150mm×4.6mm, 5μm), mobile phase is acetonitrile: water=17:83 (0.1% trifluoroacetic acid), detection Wavelength: 302nm, flow rate: 1ml / min, injection volume 20μl. Chromatographic column: SinoPak C18 5 μm. Column size: 4.6mm*200mm.
[0052] (2) Draw a standard curve
[0053] Dilute the previously prepared 110μg / ml MTX standard solution with distilled water to make solutions of 2.2μg / ml, 5.5μg / ml, 11μg / ml, 22μg / ml, 55μg / ml and 110μg / ml respectively, each concentration Inject once, each injection volume is 20 μl. Take the peak area as the ordinate, and the concentration of the standard solution as the abscissa, and perform linear regression.
[0054] (3) Sample processing method
[0055] Measure different concentrations of methotrexate with high performance liquid chromatography,...
Embodiment 3
[0056] Example 3. Stability study of methotrexate
[0057] The effects of light time, different pH values (5.5, 7.4, 8.5, 9.0), and temperature (25°C, 40°C, 80°C) on the stability of methotrexate were compared.
[0058] (1) Light stability: Take 6 small centrifuge tubes, mark them, and add 1ml of each (110μg / ml, pH7.4) methotrexate solution into them with a pipette gun, and wrap the dark group completely with tinfoil To avoid light conditions. The two groups were placed under sunlight at the same time, and samples were taken at 6h, 24h, 48h, 96h, and 144h respectively.
[0059] (2) Temperature stability: Take 9 small centrifuge tubes, add 1ml of each (110μg / ml, pH 7.4) methotrexate solution into them with a pipette gun after marking, and store them in different temperatures (25°C) in the dark. , 40°C, 80°C), and samples were taken at 6h, 24h, 48h, 72h, and 96h.
[0060] (3) pH stability: Take 12 small centrifuge tubes, add pre-prepared methotrexate solutions with differen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diffusion coefficient | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com