high titer polymyxin e 2 Sodium methanesulfonate
A technology of polymyxin and sodium methanesulfonate, applied in the field of medicine, can solve problems such as difficulty in guaranteeing the quality stability between batches of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Polymyxin E 2 Preparation of sodium methanesulfonate
[0047] (1) Purified Polymyxin Sulfate 2
[0048] Weigh an appropriate amount of industrial-grade polymyxin E sulfate (purchased from Lvkang Biochemical Co., Ltd.), dissolve it in water to make a solution containing 120 mg per 1 ml, and separate it on a chromatographic column. The chromatographic conditions are as follows:
[0049] Chromatography filler: reversed-phase microspheres with polystyrene / divinylbenzene as the skeleton, the model is XT-30, produced by Dow (DOW) Chemical Co., Ltd., USA
[0050] Column specification: 50×250mm,
[0051] Column volume (CV): 491ml
[0052] Flow rate: 20.0ml / min
[0053] Sample volume: 300ml
[0054] Elution conditions: first elute 5CV with dilute sulfuric acid solution containing 3% ethanol (use 0.05mol / L sulfuric acid to adjust the pH value of the solution to 2.4), and then use dilute sulfuric acid solution containing 8% ethanol (use 0.05mol / L sulfuric acid to ...
Embodiment 2
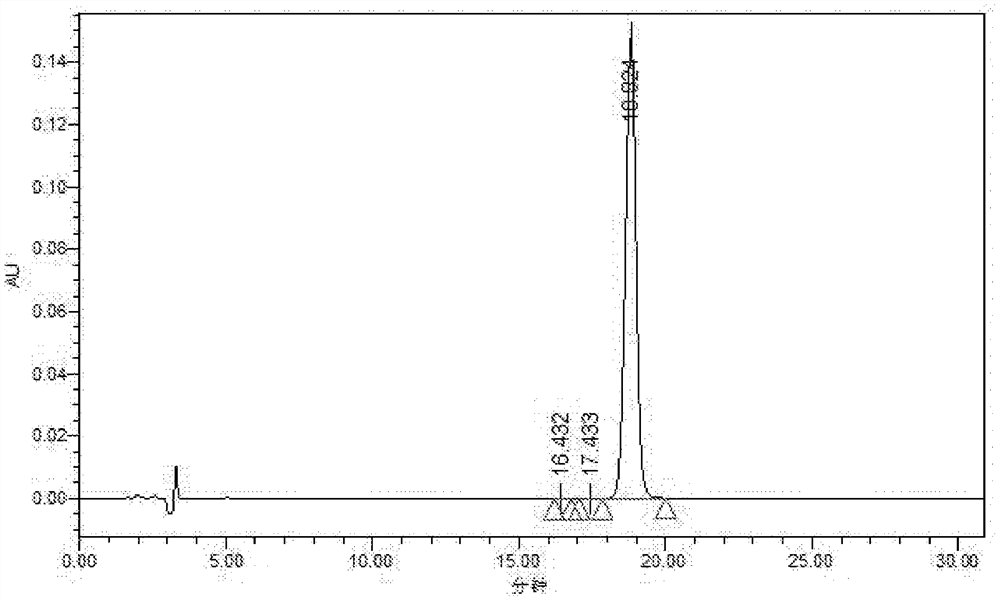
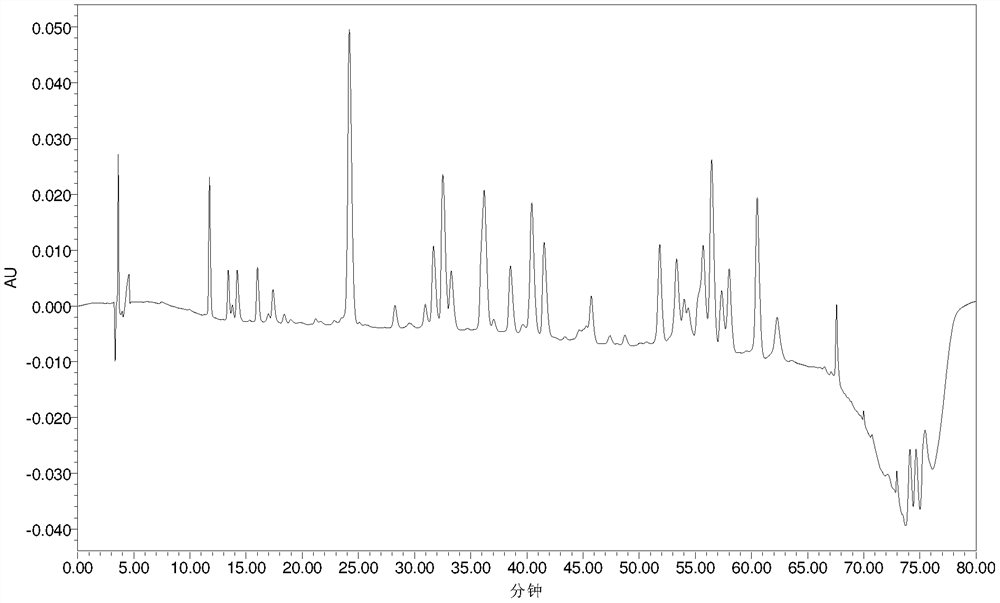
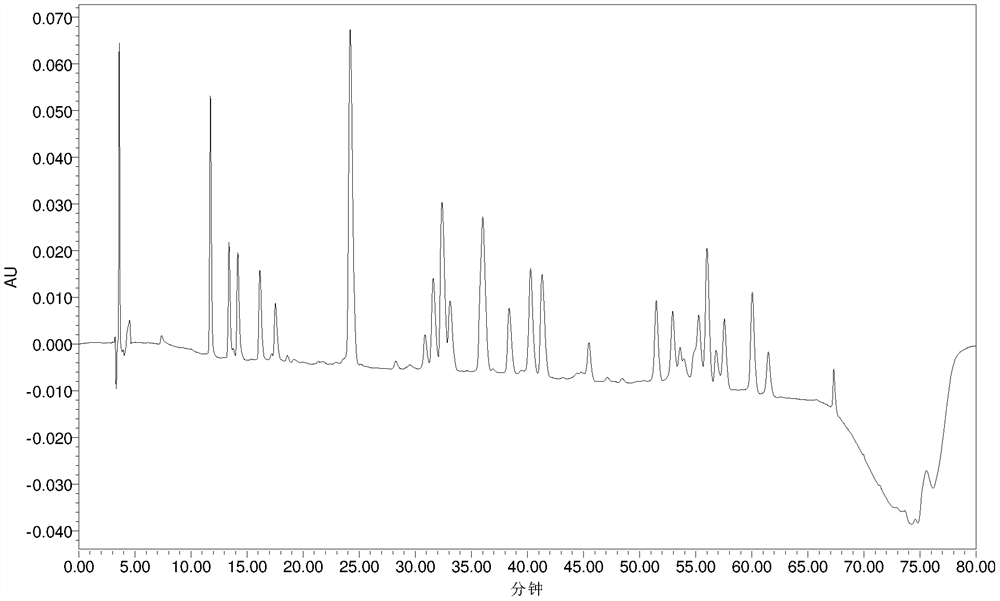
[0059] Example 2 Polymyxin E 2 Analysis of modification degree of sodium methanesulfonate
[0060] The polymyxin E that embodiment 1 prepares by LC-MS 2 Sodium methanesulfonate was used for modification analysis.
[0061] (1) LC-MS detection method
[0062] HPLC conditions are as follows:
[0063] Instrument: Shimadzu UFLC XR (LC-20AD XR pump; SIL-20AC XR autosampler; CTO-20AC column thermostat; SPD-M20A detector)
[0064] Chromatographic column: Waters Atlantis T3 (150mm×4.6mm, 3μm) 620#
[0065] Mobile phase: Phase A: 10mM ammonium formate-acetonitrile (95:5)
[0066] Phase B: 10mM ammonium formate-acetonitrile (50:50)
[0067] Linear gradient elution, the elution program is as follows:
[0068]
[0069] Flow rate: 1.0ml / min
[0070] Column temperature: 30°C
[0071] Detection wavelength: 210nm
[0072] The mass spectrometry conditions are as follows:
[0073] Instrument: AB SCIEX Triple TOF 4600
[0074] Ion source: DuoSpray TM Ion Source (ESI)
[0075] pa...
Embodiment 3
[0094] Example 3 Polymyxin E 2 Preparation of sodium methanesulfonate
[0095] With reference to the method purification polymyxin sulfate of embodiment 1 step (1) 2 , to obtain 140ml of concentrated solution, which contains 5.6g of polymyxin E sulfate 2 .
[0096] With the 140ml polymyxin E sulfate that step (1) obtains 2 Put the concentrated solution in a water bath at 25°C for 10 minutes, add 4.4ml of formaldehyde solution, adjust the pH with 2.5mol / L NaOH to keep it between 6.5-7.5, stir for 2 hours, then add 40% NaHSO 3 The solution was 16ml, and the pH was adjusted to 7.0 with 2.5mol / L NaOH, and the temperature was controlled by a water bath at 25°C for 18h. The ultrafiltration membrane (MILLIPORE company) that is 3000Da molecular weight cut-off is carried out desalting treatment by reaction solution, when the total volume remaining about 50ml of ultrafiltration intercepted liquid, add deionized water to 220ml, control feed liquid temperature in ultrafiltration proce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com