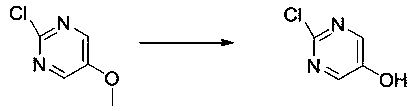
Preparation method of 2-chloro-5-hydroxypyrimidine
A technology of hydroxypyrimidine and methoxypyrimidine, which is applied in the field of preparation of 2-chloro-5-hydroxypyrimidine, can solve the problems of expensive reagents, high cost, large consumption, etc., and achieves improved purity and reaction yield and reduced cost , the effect of reducing the impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prepare a 1L four-neck flask with a stirring device and a thermometer, add 100g of 2-chloro-5-methoxypyrimidine, add 300mL of acetic acid into the reaction flask, stir well, then add 800g of 48% hydrobromic acid and 1g of methionine, and heat up to Reflux reaction for 5 hours, sample HPLC control until the end of the reaction, product content 80%, dihydroxy by-product 15%; down to room temperature, add 300mL water to the reaction solution, extract three times with 300mL dichloromethane, combine the organic phase, use saturated washed with sodium bicarbonate solution, then dried with anhydrous sodium sulfate, filtered, and the organic phase was concentrated under reduced pressure to obtain a crude product; ethanol was added to the crude product for recrystallization to obtain 64 g of a light yellow solid with a yield of 70% and a purity of 98%.
Embodiment 2
[0021] Prepare a 1L four-neck flask with a stirring device and a thermometer, add 100g of 2-chloro-5-methoxypyrimidine, add 300mL of acetic acid into the reaction flask, stir well, then add 300g of 48% hydrobromic acid and 1g of methionine, and heat up to Reflux reaction for 5 hours, sample HPLC control until the end of the reaction, product content 92%, dihydroxy by-product 5%; down to room temperature, add 300mL water to the reaction solution, extract three times with 300mL dichloromethane, combine the organic phase, use saturated washed with sodium bicarbonate solution, then dried with anhydrous sodium sulfate, filtered, and the organic phase was concentrated under reduced pressure to obtain a crude product; ethanol was added to the crude product for recrystallization to obtain 74 g of a pale yellow solid, with a yield of 80% and a purity of 98%.
Embodiment 3
[0023] Prepare a 1L four-necked flask with a stirring device and a thermometer, add 100g of 2-chloro-5-methoxypyrimidine, add 300mL of acetic acid into the reaction flask, stir well, then add 153g of 48% hydrobromic acid and 1g of methionine, and heat up to Reflux reaction for 5 hours, sample HPLC control until the end of the reaction, product content 96%, dihydroxy by-product 0.5%; cool down to room temperature, add 300mL water to the reaction solution, extract three times with 300mL dichloromethane, combine organic phases, use saturated washed with sodium bicarbonate solution, then dried with anhydrous sodium sulfate, filtered, and the organic phase was concentrated under reduced pressure to obtain a crude product; ethanol was added to the crude product for recrystallization to obtain 82 g of a light yellow solid, with a yield of 91% and a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com