Preparation method and product of ethylenediamine
A technology of ethylenediamine and ethylenediamine hydrochloride, which is applied to the preparation of amino compounds from amines, the purification/separation of amino compounds, organic chemistry, etc., can solve the problems of difficult operation, cumbersome operation, and high toxicity of solvents, and achieve The effect of reducing the difficulty of the process, improving the economic efficiency and reducing the workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1.1 Reagents and instruments
[0037] The test drugs and test equipment used in this embodiment are shown in Table 1 and Table 2.
[0038] Table 1 Experimental drugs
[0039]
[0040] Table 2 Experimental equipment
[0041]
[0042] 1.2 Composition and property analysis of ethylenediamine hydrochloride
[0043] First of all, it is necessary to analyze the approximate content of ethylenediamine in the sample salt, estimate and compare the difference in economic benefits between the finished product ethylenediamine and the sample salt, and provide an economic basis for the entire ethylenediamine hydrochloride reuse project. At the same time, it is necessary to do some property analysis on the sample salt to find the optimum temperature for its dissolution so that it can directly react with solid sodium hydroxide.
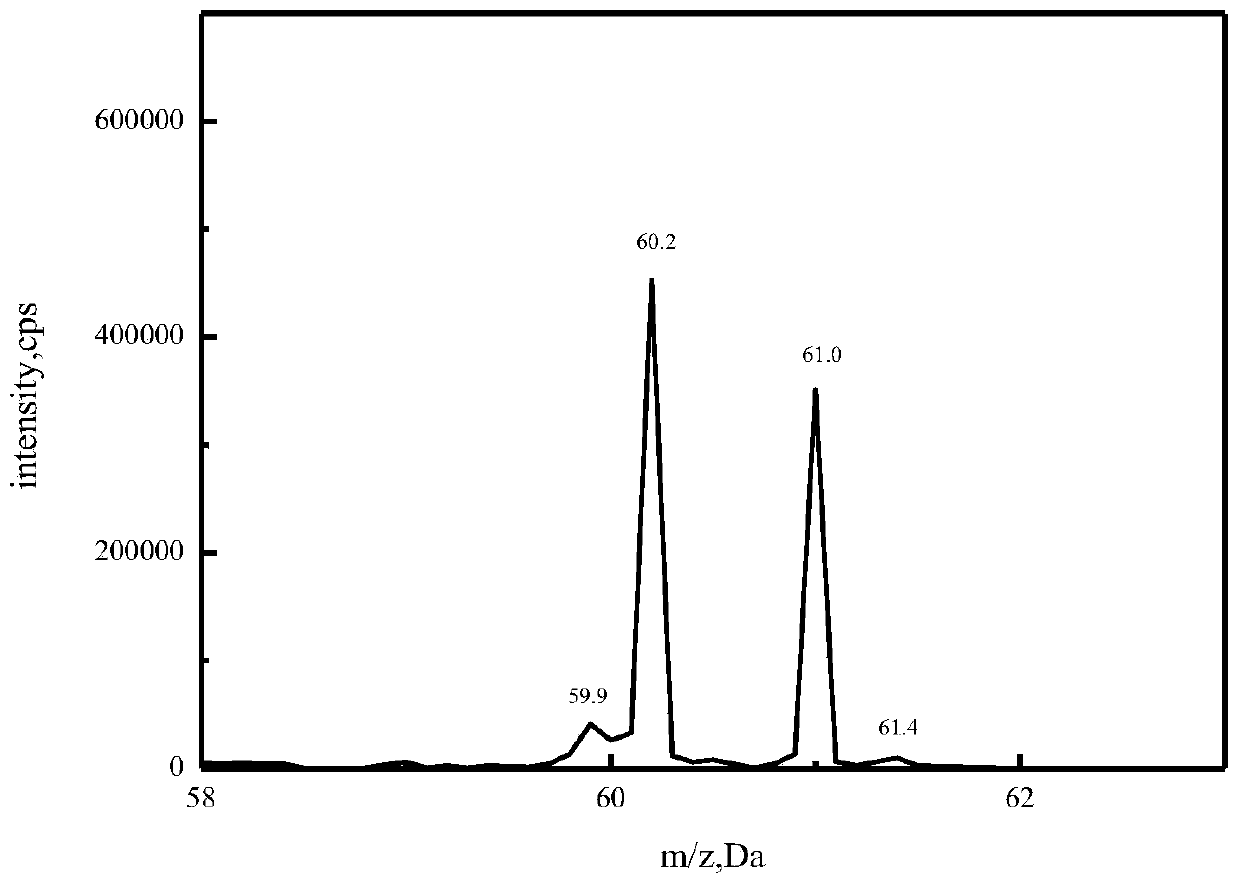
[0044] Because ethylenediamine is a dibasic base, the ethylenediamine hydrochloride sample contains C 2 h 8 N 2·HCl and C 2 h 8 N 2 · 2HCl two h...
Embodiment 2
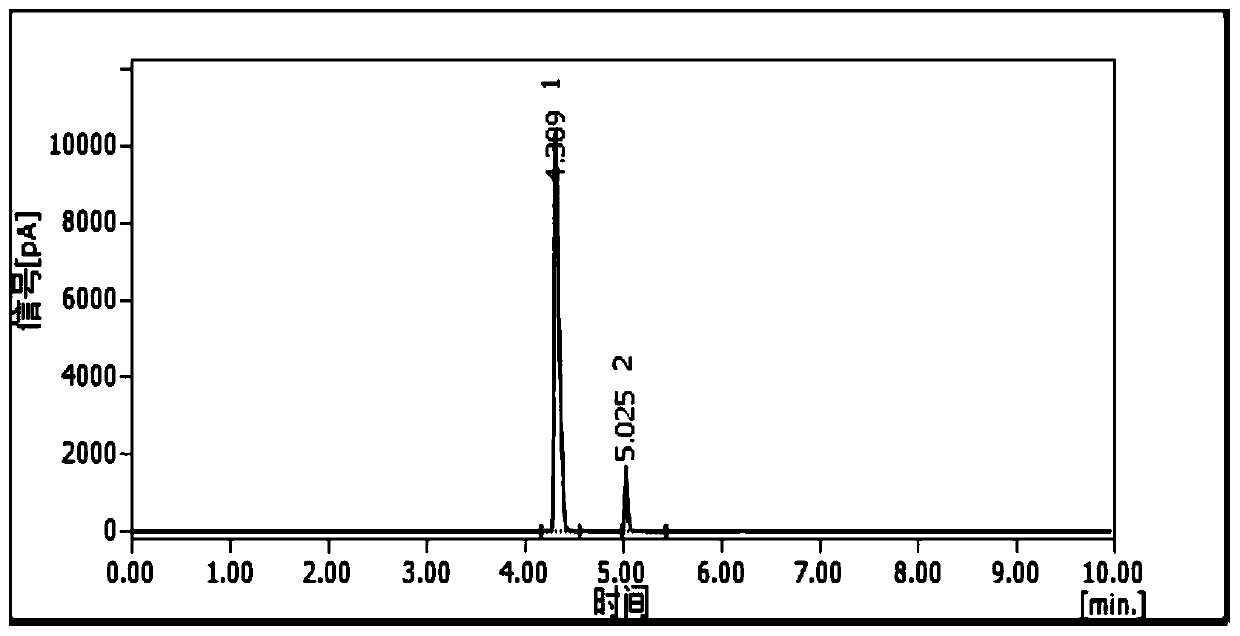
[0095] In Example 1, the highest purity of 97% ethylenediamine was obtained, but this purity ethylenediamine cannot be used in some specific processes, so it is necessary to explore a more efficient dehydration method.
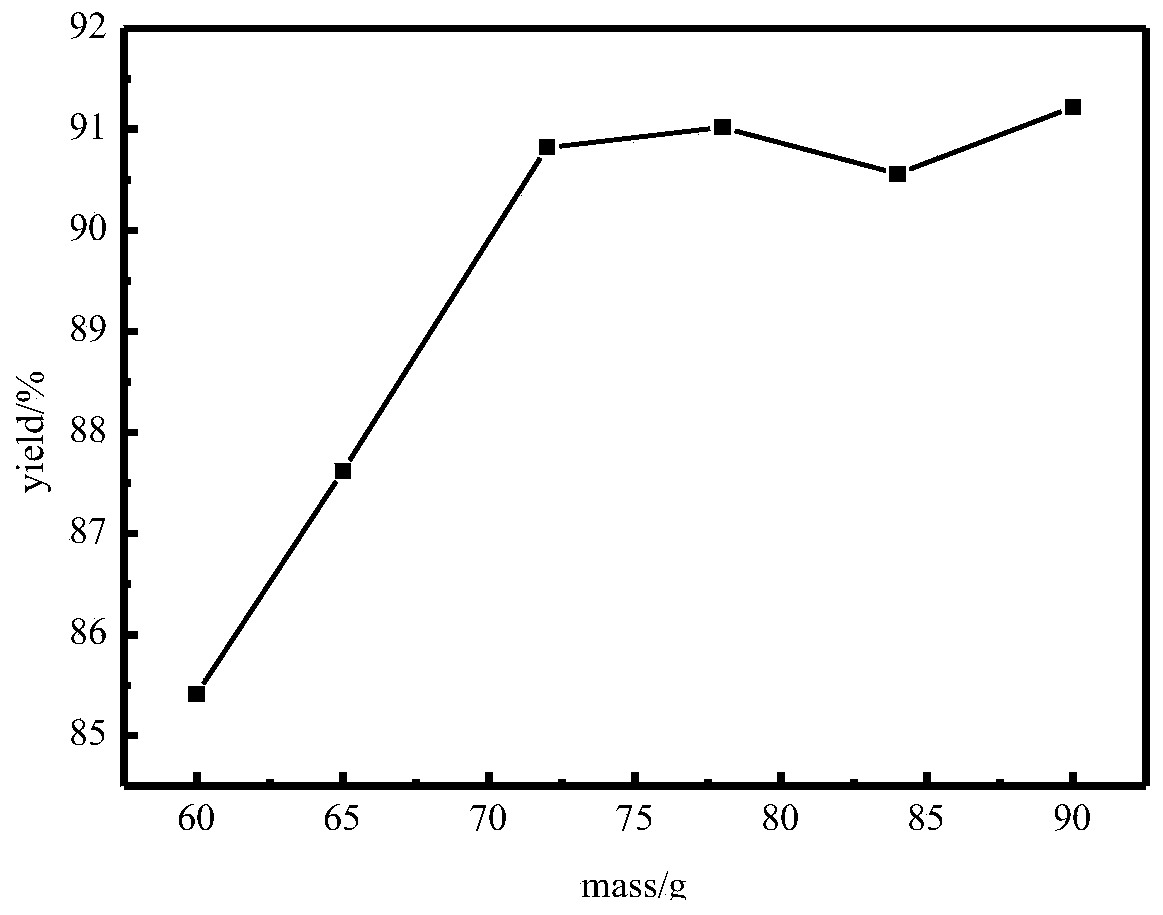
[0096] In this example, 200 g of ethylenediamine with a water content of 16.10% prepared in Example 1 is directly added to the still of the rectification column, the extractant is 600 g of 1,4-butanediol, and the initial value of the reflux ratio is 0.5. Vacuum distillation operation. Record the water output at the top of the tower and the content of ethylenediamine and extractant at different temperatures and time periods as shown in Table 8.
[0097] Table 8 batch distillation experimental data
[0098]
[0099] It can be seen from Table 8 that substances start to be extracted from 65°C after feeding, and between 65°C and 95°C, there is a lot of water and a part of ethylenediamine is extracted at the same time. The process takes 1.5h, and the process is ...
Embodiment 3
[0101] The difference between this example and Example 2 is that the oil bath is heated to 60°C, and the rest of the steps and parameters are the same as in Example 1. The mass of the crude product finally obtained in this example is 75g, and the mass of the crude product ethylene glycol is 75g. The water content is 16.1%, then the yield of ethylenediamine is 75×83.90% / 140×60.86%×100%=73.85%. The crude product of ethylenediamine was dehydrated and purified by batch rectification, and the treatment steps were the same as in Example 1. The purity of the finally obtained ethylenediamine was 97.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com