Recombinant virus-like particle expressed based on inclusion body form, preparation method and application thereof
A technology of recombinant virus and inclusion body, applied in the field of biomedicine, can solve the problems of no biological activity, wrong structure, difficult to remove, etc., and achieve the effect of simple and easy to operate preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
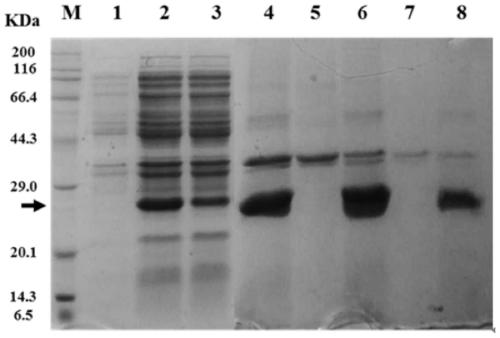
[0092] Expression and Identification of Recombinant Hepatitis B Virus Core Antigen Fused with Other Antigens
[0093] The specific operation method is:
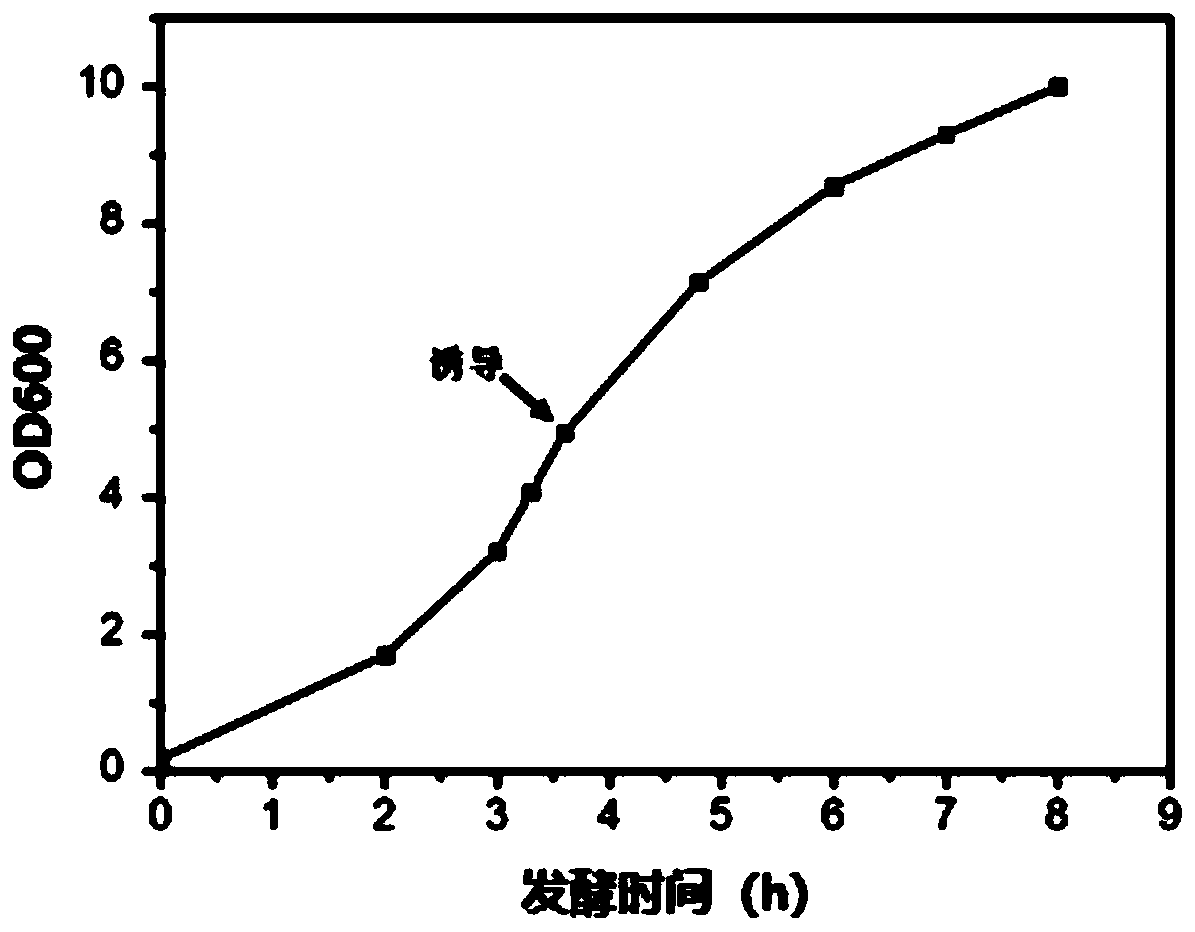
[0094] (1) Insert a major histocompatibility complex MHC class II antigen (the amino acid sequence is SEQ ID NO.3: AELVHFLLLKYRAR) in the human melanoma-related gene MAGE A3 gene into the hepatitis B virus core antigen HBc MIR by means of genetic engineering Region (inserted between HBc 78aa-79aa), ligated into plasmid pET28a, named pET28a-HBc-MAGE3II. The recombinant plasmid was subcloned into competent Escherichia coli cells BL21(DE3), and the amino acid sequence of HBc-MAGE3II is shown in SEQ ID NO.4. Inoculate to 20L fermenter for fermentation, wait for OD 600 At 5-8 hours, add 0.1mM isopropylthiogalactopyranoside (IPTG) to induce 4h to end the fermentation, and draw the thalline growth curve, such as figure 1 shown.
[0095] SEQ ID NO.4:
[0096] Mdidpykefgasvellsflpsdffpsirdlldtasalyrealespehcsphhtalrqailcwgelmnlat...
Embodiment 2
[0103] dissolution of inclusion bodies
[0104] The specific operation method is:
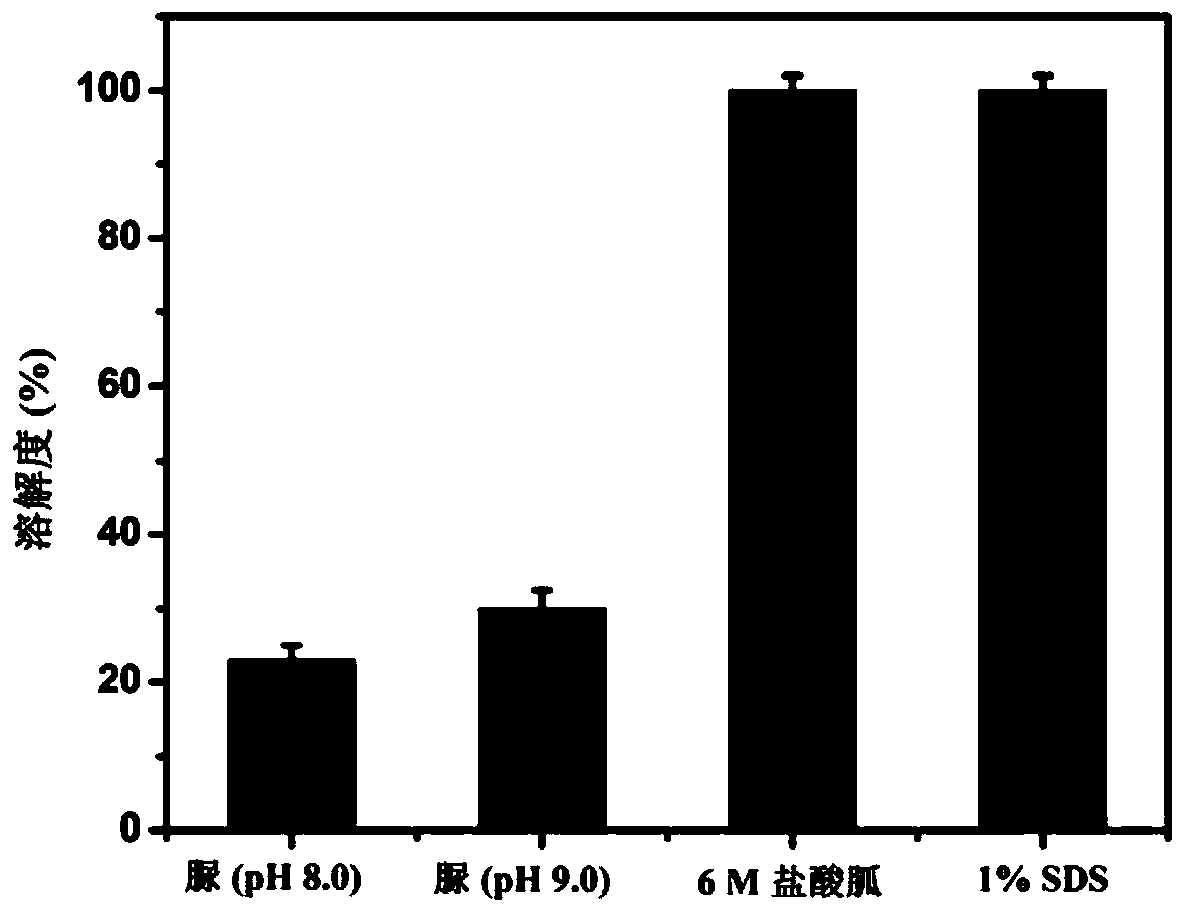
[0105] (1) Weigh 0.1 g of the inclusion bodies obtained in Example 1, add 1 mL of denaturant containing 8M urea, 6M guanidine hydrochloride or 1% SDS respectively, shake overnight at room temperature, collect the supernatant by centrifugation to measure the protein concentration and calculate the dissolution rate. The result is as image 3 shown.
[0106] (2) Add the inclusion bodies after washing in Example 1 to the dissolving buffer containing 20mM Tris-HCl, 1% SDS, and 0.5% β-mercaptoethanol at pH 8.0 at a ratio of 1g:1mL. The inclusion bodies are excessive and the solubility reaches saturation , using the Bradford method to measure the protein concentration, about 20mg / mL.
[0107] This example also explores the effects of different types of dissolving solvents and subsequent treatments on the secondary structure of proteins. The protein secondary structure of the processed samples was ...
Embodiment 3
[0112] extracellular assembly of inclusion bodies
[0113] The specific operation method is:
[0114] (1) Dilute the inclusion bodies dissolved in SDS obtained in Example 2 with pH 8.0 containing 15-25mM Tris-HCl and 0.4%-6% β-mercaptoethanol at 4°C. After diluting 100 times overnight, add 2-Methyl-2,4-pentanediol with a final concentration of 1M was reacted at 20°C for 2 days, and then placed at 4°C for 3 days after desalting. The desalting column used for desalting was G25 desalting column, and the buffer used for desalting was pH 7.4 20 mM Tris-HCl solution.
[0115] This example also explored the effect of different dilution factors on the protein. The specific method is: prepare the recombinant hepatitis B virus core antigen in the form of inclusion bodies fused with other antigens according to the method of Example 1-3, and wash the inclusion bodies. The inclusion bodies were dissolved with 1% SDS solution, diluted 10 times, 20 times, 50 times and 100 times respectivel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com