Micro effervescent tablet with seaweed and baking soda and preparation method and application of micro effervescent tablet
A technology of baking soda and microbubbles, which is applied in the direction of medical formulas, medical raw materials derived from algae, medical preparations containing active ingredients, etc., can solve the problems of bad flavor, alkalosis, high price, etc., and achieve rich mineral content, Less side effects, good flavor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
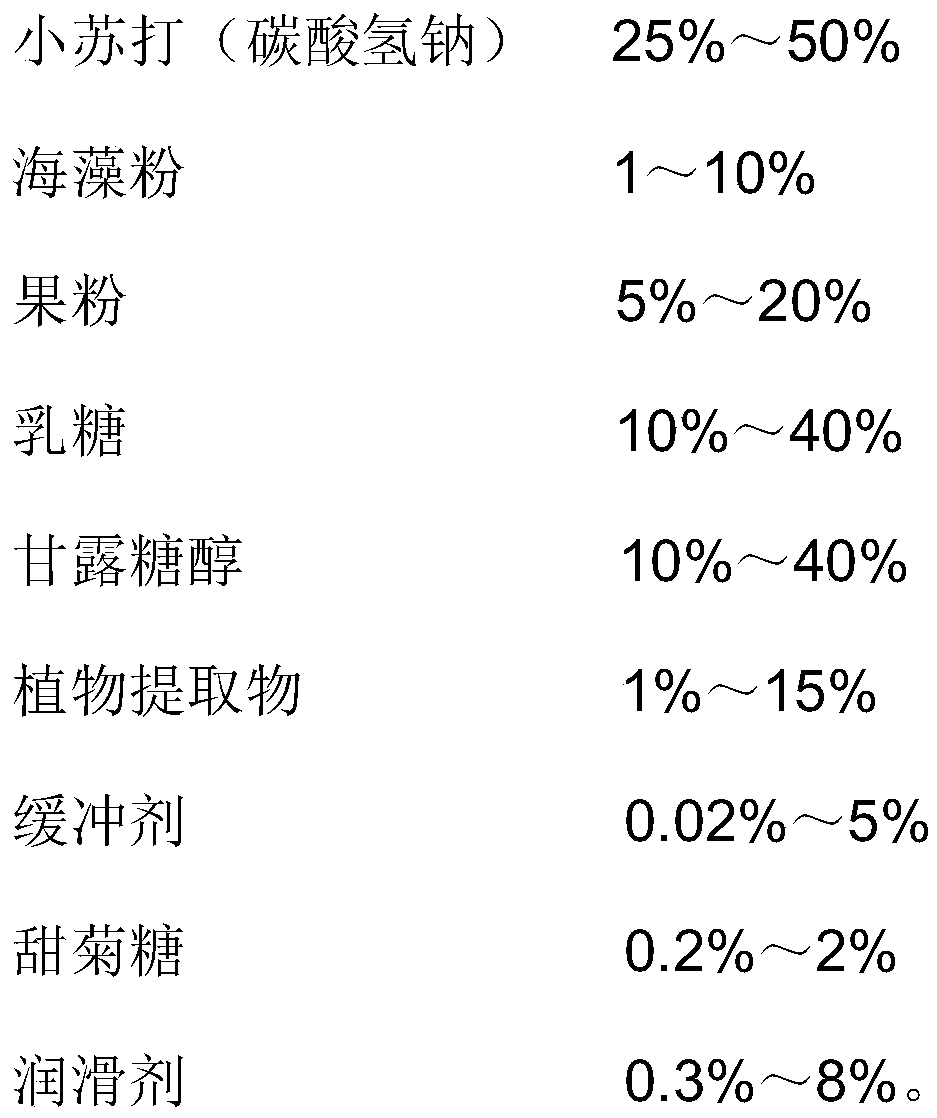
[0031]
[0032]
[0033] Wherein, the plant extract is a mixture of dandelion, Sophora japonica and Pachyphyllum leaf extracts, the weight ratio of each component is: Dandelion extract: Sophora japonica extract: Pachyphyllum pachyrhiza extract=1:1:1: 1. Provided by Shaanxi Senfu Natural Products Co., Ltd.
[0034] Production method: 1. Baking soda granulation: granulate baking soda with 85% ethanol solution;
[0035] The weight ratio of baking soda to solvent is 5.7:1;
[0036] 2. Weigh the granulated baking soda and other materials (except polyethylene glycol), pass through a 20-mesh sieve, and mix for 15 minutes;
[0037] 3. After polyethylene glycol passes through an 80-mesh sieve, mix it with 2 for 5 minutes;
[0038] 4. Press the mixed powder of 3 into tablets, 600mg per tablet;
Embodiment 2
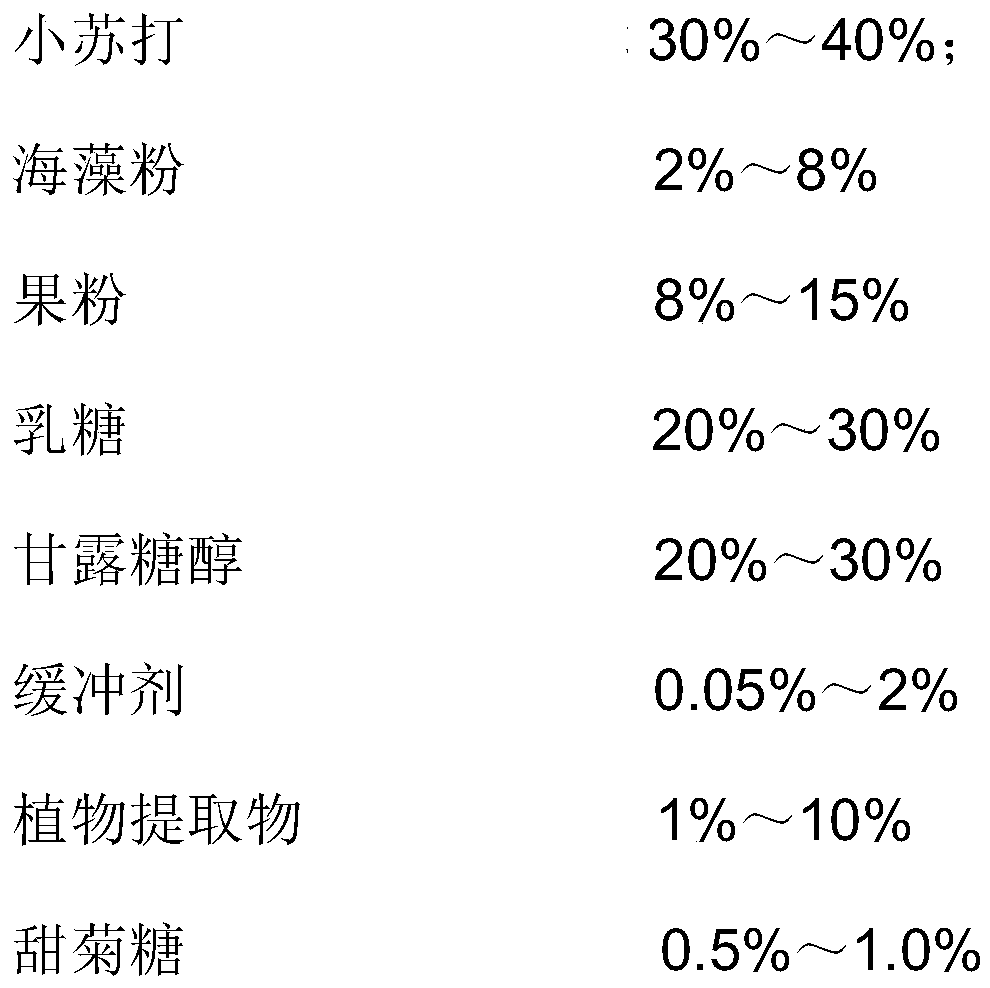
[0040]
[0041]
[0042] Wherein, the plant extract is a mixture of dandelion, Sophora japonica and Paebrania leaf extracts, the weight ratio of each component is: dandelion extract: Sophora japonica extract: Pachyphyllum phallus leaf extract=1:1:1,
[0043] Production Method:
[0044] 1. Baking soda granulation: Baking soda is granulated with a 5% maltodextrin solution by weight;
[0045] The weight ratio of baking soda to solvent is 9:1;
[0046] 2. Weigh the granulated baking soda and other materials (except sodium stearyl fumarate), pass through a 20-mesh sieve and mix for 15 minutes;
[0047] 3. Sodium stearyl fumarate passed through a 40-mesh sieve, mixed with 2 for 5 minutes;
[0048] 4. Press the mixed powder of 3 into tablets, 600mg per tablet;
Embodiment 3
[0055] Clinical Observation:
[0056] (1) Clinical data
[0057] A clinical trial was conducted on 245 patients with gouty arthritis. These patients all had symptoms of joint pain and swelling in varying degrees, and the course of the disease was 1-3 years. There were 155 males and 90 females. The treatment group included 130 cases (Example 1: 70 persons; Embodiment 2: 60 persons), and the control group 115 cases (Comparative Example 1: 60 persons; Comparative Example 2: 55 persons).
[0058] (2) Treatment method
[0059] The control group was given comparative example 1 and comparative example 2; the treatment group took orally the embodiment 1 and embodiment 2 of the present invention, 2 times a day, 2 tablets each time; cycle 15 days
[0060] (3) Judgment criteria for curative effect:
[0061] Significantly effective: no pain in the knee joint, no tenderness around the joint, no swelling of the joint, and the range of motion of the joint is 130°.
[0062] Effective: Sl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com