Exosome sustained release system and construction method and application thereof
A construction method and technology of exosomes, applied in the direction of prostheses, liquid delivery, pharmaceutical formulations, etc., can solve the problems of short retention time, retention time of no more than 24 hours, and increased treatment costs, so as to improve treatment efficiency and long-term release , to solve the effect of short residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Preparation of hyaluronic acid solution loaded with bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos)
[0083] Extraction of BMSC-Exos: Take 5 bottles of human bone marrow mesenchymal stem cells whose cell growth area is 60%-70% of T75, replace with 15mL serum-free medium and continue to culture for 2 days. These media were collected and divided into ten 50 mL centrifuge tubes. Centrifuge at speeds of 300g, 2,000g, and 10,000g for 20 minutes respectively to remove impurities such as cells, cell debris, and organelles in the culture medium. Then centrifuge at 100,000 for 1 h, and aspirate the supernatant, and keep about 200 μL of the lower liquid in each tube. Exosomes were identified by transmission electron microscopy and immunoblotting of surface proteins.
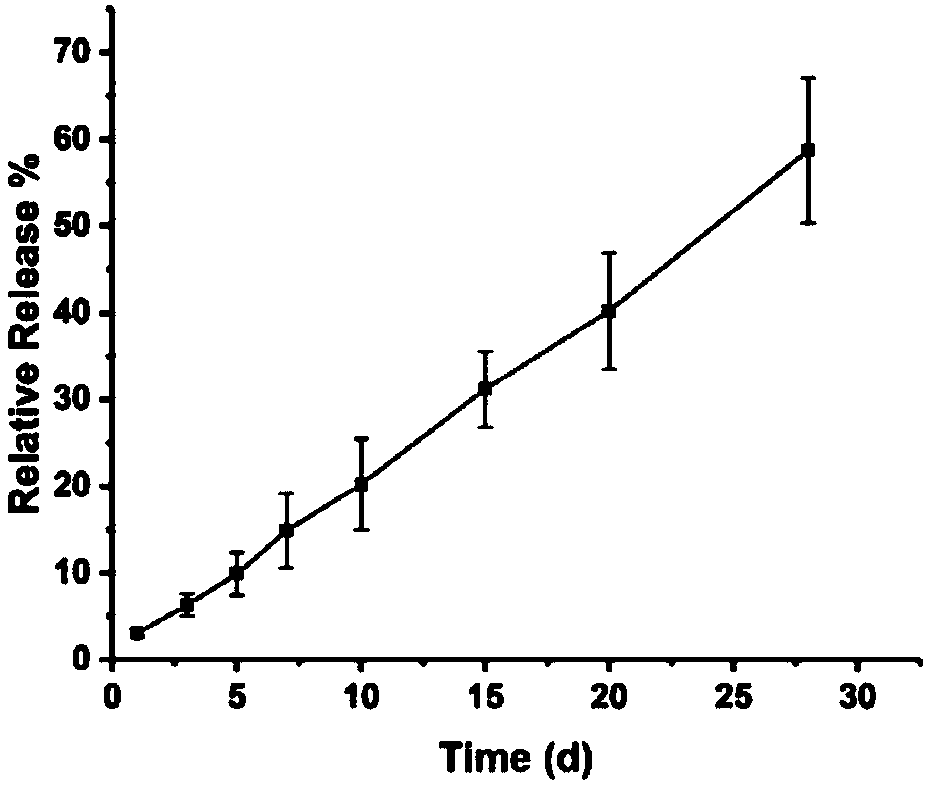
[0084] Subsequently, 200 μL of exosomes were dispersed in 1 ml of physiological buffer. The concentration of exosomes in the exosome suspension was measured by a nanoparticle analyzer to be 1 × 10 ...
Embodiment 2
[0086] Construction of photocrosslinked hydrogel exosome sustained-release system loaded with induced pluripotent stem cell-derived exosomes (iPS-Exos)
[0087] Culture of human induced pluripotent stem cells (iPSCs) and extraction and identification of exosomes
[0088] A layer of embryonic stem cell Matrigel (ESC-Qualified BD Matrigel, BD Sparks, MD, USA), iPSCs were moved into the dish, and mTeSR1 serum-free medium (StemCell Vancouver, BC, Canada), in an incubator (37°C, 5% CO 2 , saturated humidity) culture, and collect the culture medium changed every day. Filter the medium through a 0.22-micron pore-size filter membrane and centrifuge at 10,000 g at 4°C for 30 minutes to remove cell debris; use an ultrafiltration tube with a number-average molecular weight of 100 KD, centrifuge (3,500 g, 15 min) to intercept exosomes in the concentrated supernatant, and obtain exosomes. Secretion concentrate; transfer the concentrate to a 30% sucrose / heavy water density pad (1.210 g / c...
Embodiment 3
[0093] Construction of hyaluronic acid membrane loaded with embryonic stem cell-derived exosomes (ES-Exos)
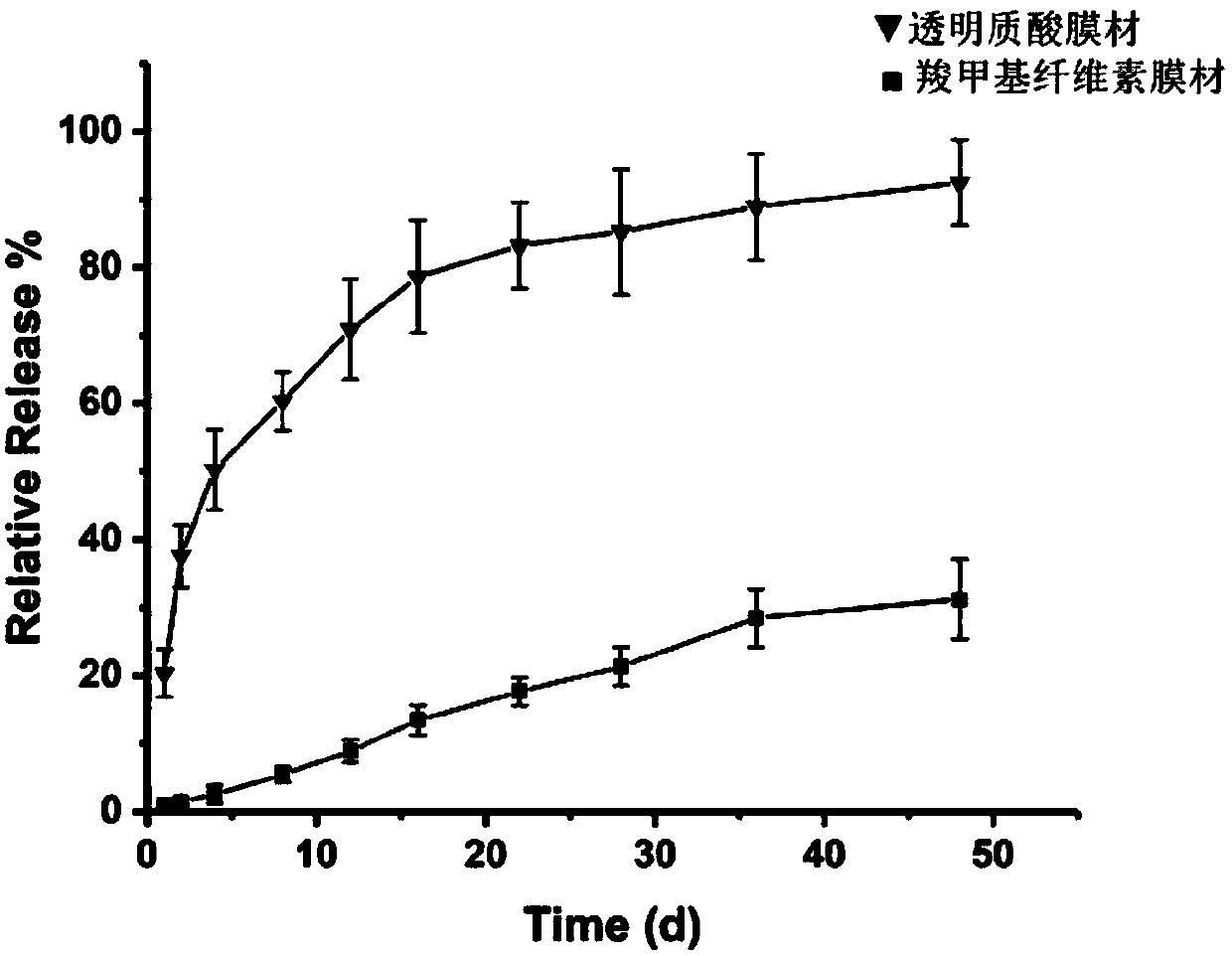
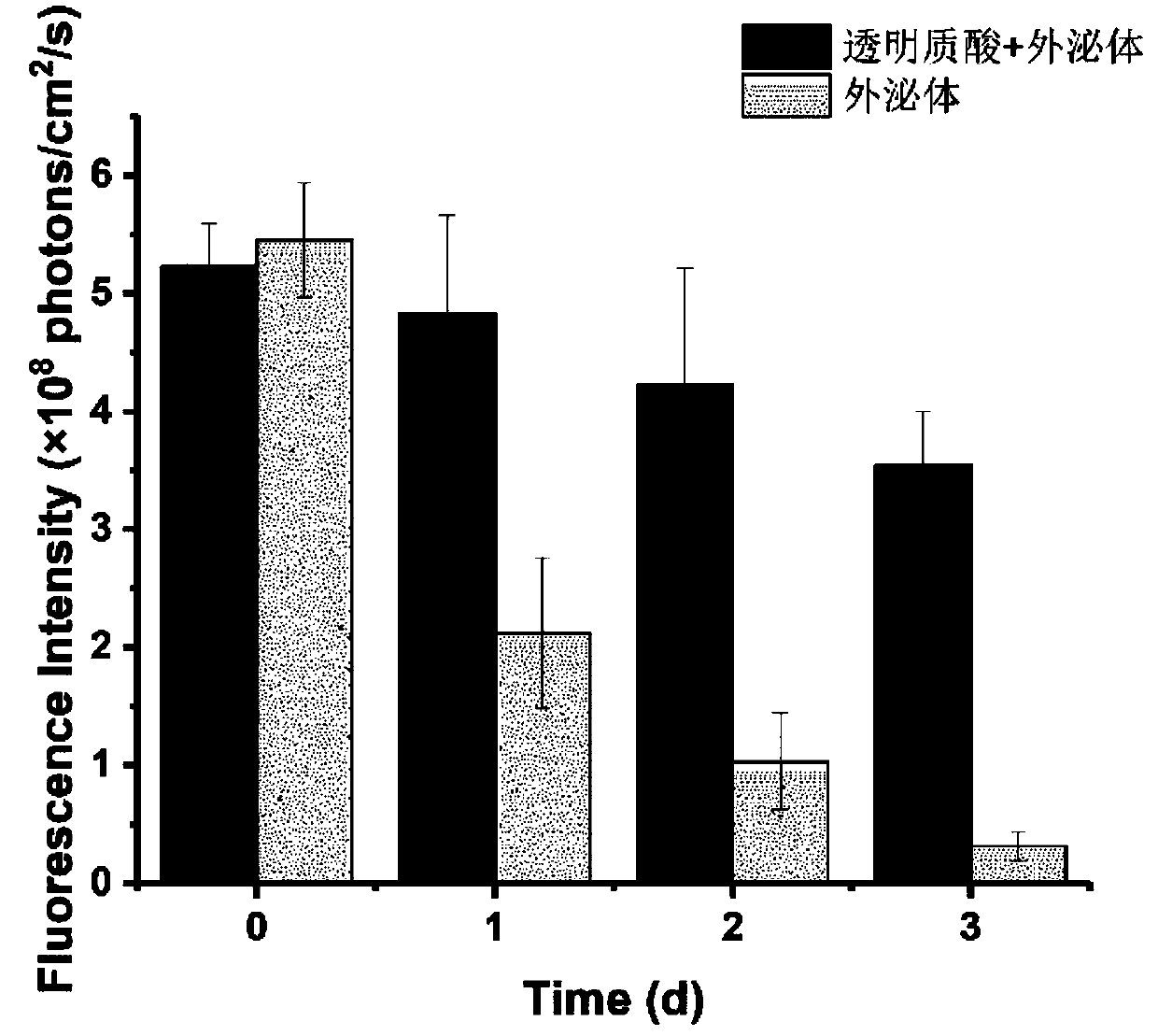
[0094] The induced pluripotent stem cell-derived exosomes (iPS-Exos) extracted in Example 2 were dispersed in 1 mL of distilled water, and the concentration of the exosome suspension measured by a nanoparticle analyzer was 1×10 10 / mL. Accurately weigh 0.05 g of hyaluronic acid with a number average molecular weight of 130 KDa and 0.05 g of gelatin, dissolve them in 9 mL of distilled water, and mix them with the iPS-Exos suspension. Then 500 μL of the above solution was evenly spread on the polytetrafluoroethylene base plate, and the base plate was placed in a constant temperature drying oven at 40 °C for 24 hours to obtain a hyaluronic acid-gelatin film loaded with iPS-Exos.
[0095] Further, the same iPS-Exos-loaded carboxymethyl fiber-gelatin film as the above-mentioned film material was prepared according to the same method. Soak the two membranes in 2mL of physio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Shear modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com