Pharmaceutical composition for treating and preventing obesity and non-alcoholic fatty liver disease
A non-alcoholic, fatty liver technology, applied in the hawthorn flavonoid-total tanshinone composition, the application field in the preparation of drugs and/or health care products for preventing and/or treating non-alcoholic fatty liver, can solve the problem of uneconomical, easy-to-obtain, The efficacy of synergistic gain needs to be studied, and the treatment effect of a single component is not discussed, so as to achieve the effect of streamlining raw materials, relieving excessive weight gain, and reducing fat accumulation in liver tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
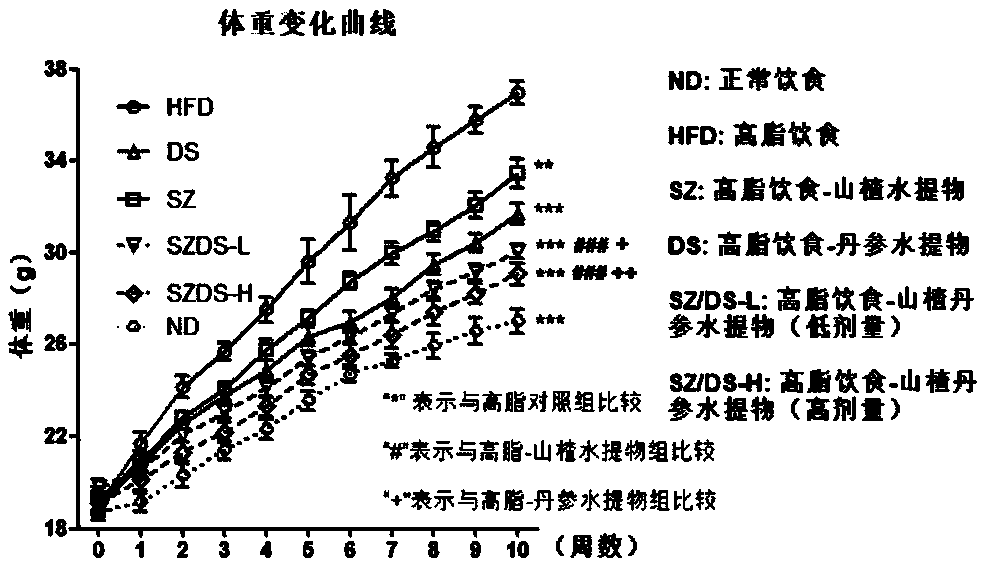
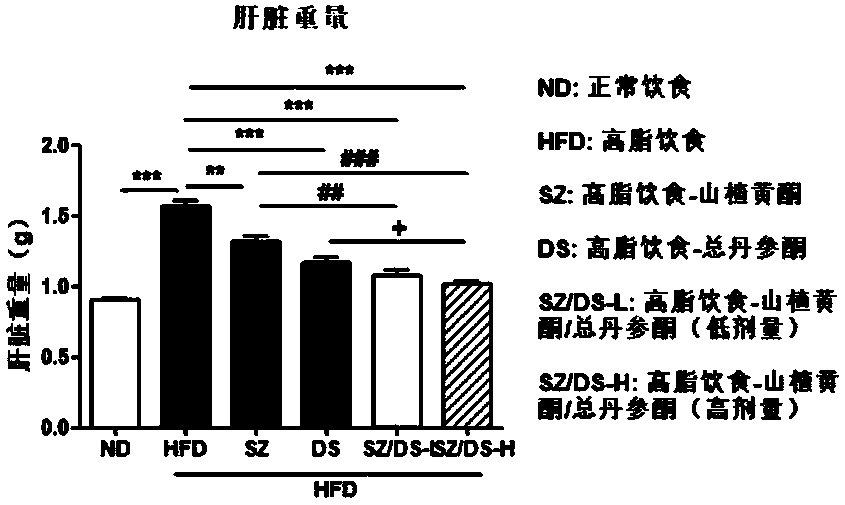
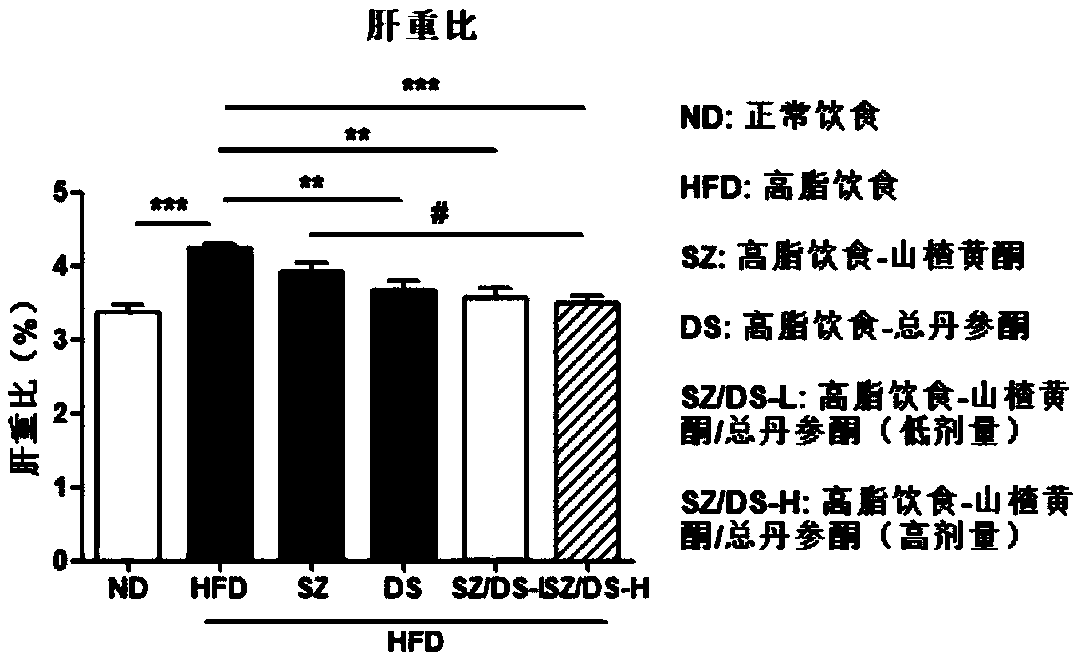
[0062] Example 1 Hawthorn flavonoids-total tanshinone for the prevention and treatment of non-alcoholic fatty liver and obesity caused by high-fat diet model
[0063] 1. Instruments and materials
[0064] Main instruments: electronic balance (Shanghai METTLER TOLEDO); full-wavelength microplate reader (Thermo Company, USA); centrifuge (Eppendorf Company, Germany); low-speed desktop centrifuge (HKUST Zhongjia).
[0065] Drugs and reagents: 60% high-fat feed (TP2330055A) and its control feed (TP 2330055AC, Nantong Trophy Feed Technology Co., Ltd.); 80% hawthorn flavonoids, 40% total tanshinone (Shaanxi Senlang Biochemical Co., Ltd.); ALT , AST, TC, TG detection kits (Nanjing Jiancheng Institute of Bioengineering); other reagents were of analytical grade.
[0066] 2. Preparation of hawthorn flavonoids-total tanshinone composition
[0067] According to the dosage, the hawthorn flavonoids are 50, 100 mg / kg; the total tanshinone is 50, 100 mg / kg. The estimated dosage of the purch...
Embodiment 2
[0093] Example 2: Preparation of tablets and capsules comprising 11-99% hawthorn flavonoids-total tanshinone:
[0094] Take hawthorn flavonoids-total tanshinone dry powder, pass through a 60-100 mesh sieve for later use. (1) Add one or several kinds of excipients commonly used in tablets; compress into tablets by a tablet machine. Each tablet contains no less than 110 mg of components, and the weight of the tablet can be adjusted according to the drug loading.
[0095](2) Add one or several kinds of auxiliary materials commonly used in capsules, granulate, granulate, and pack into capsules. Each capsule contains no less than 110 mg of components, and the weight of the capsule can be adjusted according to the drug loading. See Table 5. Suggested dosage: 6 capsules per day, 2 capsules per meal, 110-220mg extract per capsule; daily intake is 660-1320mg. Or take 3 capsules per day, 1 capsule per meal, 220-440mg extract per capsule; the daily intake is 660-1320mg. Or take 2 ca...
Embodiment 3
[0105] Example 3: Preparation of granules and suspensions containing 3-99% hawthorn flavonoids-total tanshinone: taking 10g / bag as an example, the specific dosage form specification (weight / bag) can be adjusted according to the drug loading. Take hawthorn flavonoids-total tanshinone dry powder, pass through a 60-100 mesh sieve for later use. Take 330-1320g of the above-mentioned dry powder, arbitrarily proportioning 8680-9670g of auxiliary materials; make the total weight 10000g. Add a small amount of solvent and mix well to make granules, dry, pack one bag per 10g; 1000 bags in total. The excipients include, but are not limited to: sweeteners, powdered sugar, dextrin, β-cyclodextrin, and effervescent disintegrants. The prepared hawthorn flavonoid-total tanshinone granule contains 3.3-13.2% of active ingredients in each bag. Suggested dosage: take 1-2 bags per day, with a daily intake of 660-1320mg. See Table 6. On the premise of considering different dosage forms, it is e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com