Method for preparing beta-arylamino alcohol drugs such as tulobuterol, clorprenaline, dichloroisoprenaline and sotalol
A technology of arylamino and alcohols, applied in the field of organic synthesis, can solve the problems of complex post-processing, poor applicability, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
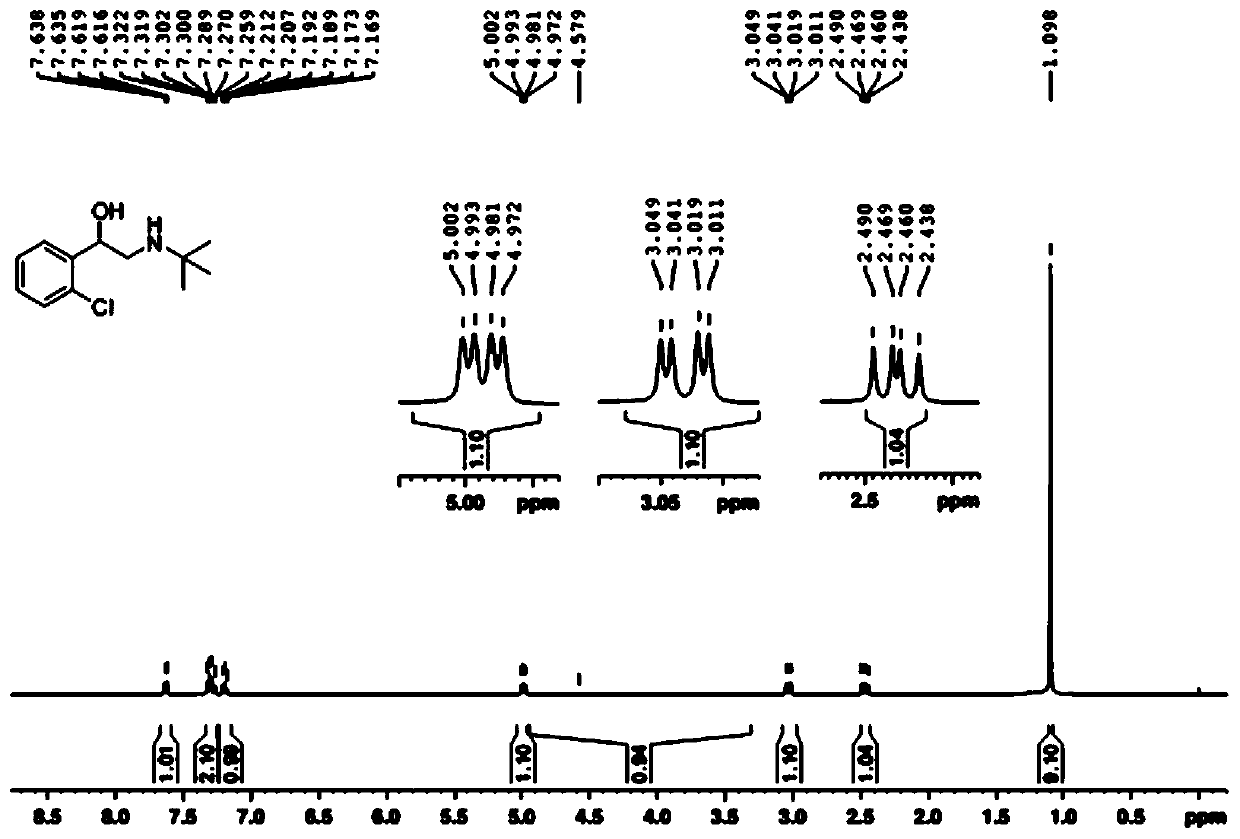
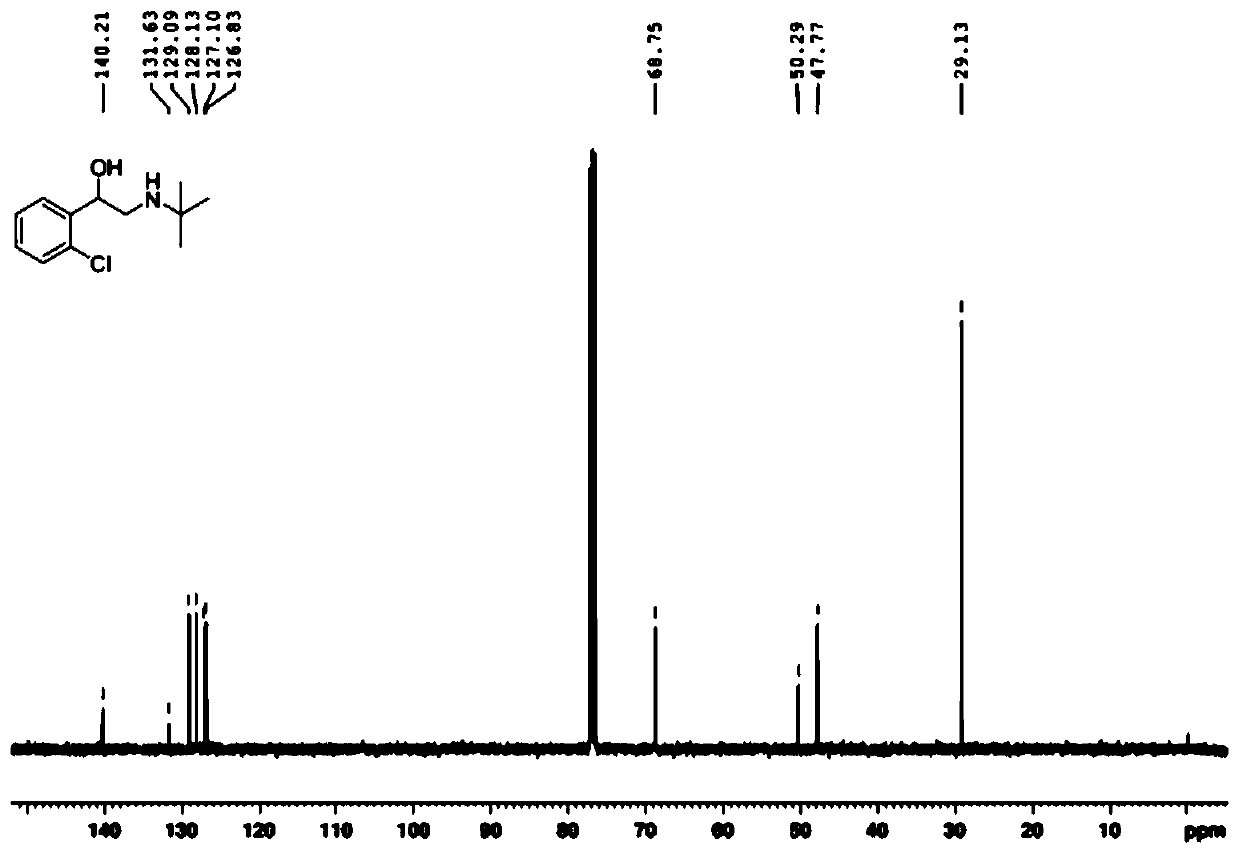
[0086] Example 1 : Synthesis of Tulobuterol in One Pot
[0087] Add o-chloroacetophenone (309.2 mg, 2.0 mmol), iodine (152.3 mg, 0.6 mmol) and dimethyl sulfoxide (5 mL) to a 50 mL pear-shaped bottle, and react at 120°C in an oil bath. TLC detects the reaction Detected every 20 minutes until the raw materials disappeared completely, and then separated and extracted with ethyl acetate and saturated brine to obtain 2-(2-chlorophenyl)-2-oxoacetaldehyde.
[0088] Transfer the prepared 2-(2-chlorophenyl)-2-oxoacetaldehyde to a 50mL pear-shaped flask, add 5 mL of 1,2-dichloroethane, add tert-butylamine (175.5mg), 25℃ Reaction for 2h at temperature. Then sodium borohydride (90.8 mg) was added to the system several times in small amounts, and reacted at 0°C for 2 hours. After the solvent was removed by rotary evaporation, it was separated by silica gel column (eluent, petroleum ether: ethyl acetate = 5:1-0:1) to obtain 332.5 mg of white solid.
[0089] The melting point of the white solid...
Embodiment 2
[0090] Example 2 :Synthesis of tulobuterol by gram reaction
[0091] Add o-chloroacetophenone (10.0g, 64.7mmol), iodine (4.93g, 19.4mmol) and dimethyl sulfoxide (65mL) to a 200mL pear-shaped flask and heat the reaction in an oil bath at 120℃. TLC detects the reaction. Detected every 20 minutes until the raw materials disappeared completely, and then extracted with ethyl acetate and saturated brine, and concentrated to obtain 2-(2-chlorophenyl)-2-oxoacetaldehyde.
[0092] Add 1,2-dichloroethane (65 mL) as a solvent and tert-butylamine (5.9 g, 77.6 mmol) to the system, and react at 25° C. for 2 h. Then sodium borohydride (2.9 g, 77.6 mmol) was added to the system several times in small amounts, and the reaction was carried out at 25° C. for 2 h. After the solvent was removed by rotary evaporation, it was separated by silica gel column (eluent, petroleum ether: ethyl acetate = 5:1-0:1) to obtain 6.7 g of white solid.
[0093] The melting point was measured by capillary melting point ...
Embodiment 3
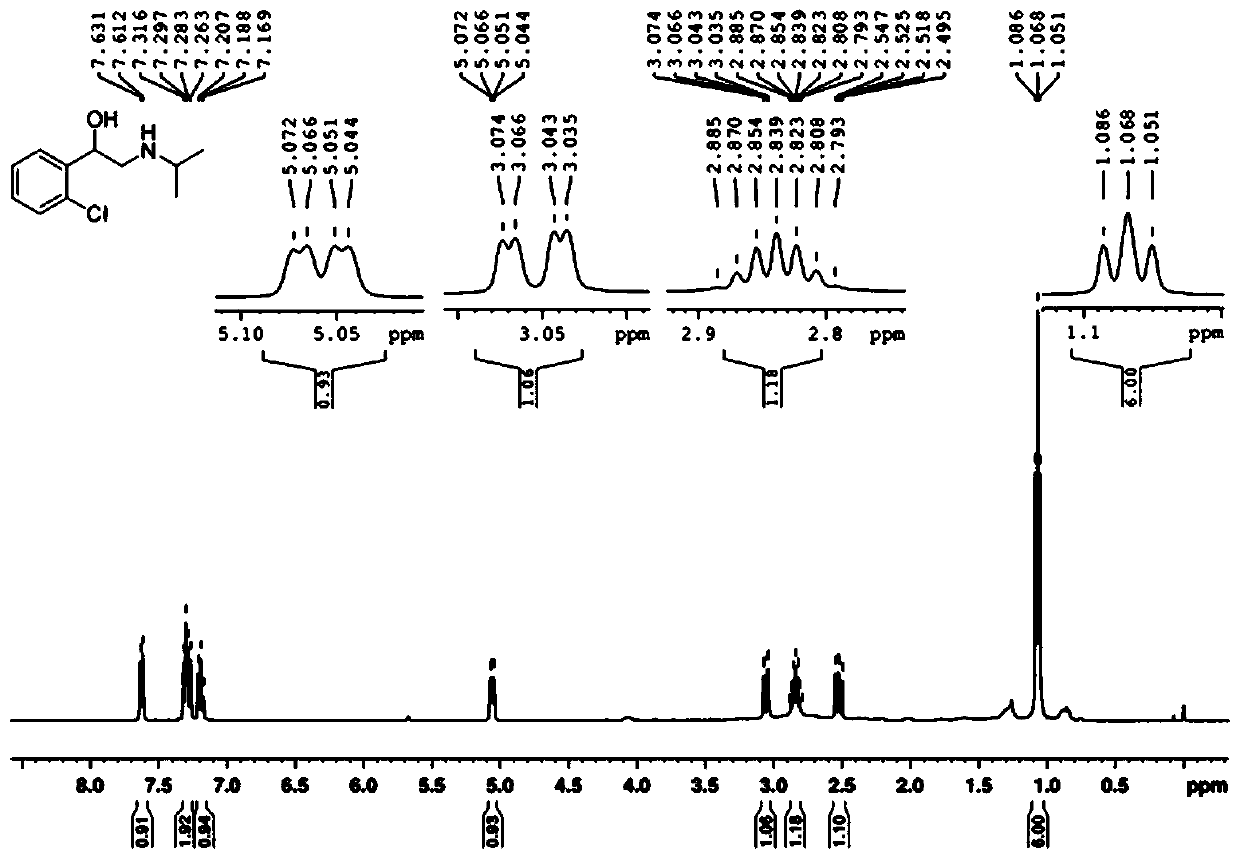
[0094] Example 3 :Synthetic Cloprenaline
[0095] Add o-chloroacetophenone (309.2 mg, 2.0 mmol), iodine (152.3 mg, 0.6 mmol) and dimethyl sulfoxide (5 mL) to a 50 mL pear-shaped flask, and react at 120°C in an oil bath. TLC detects the reaction Detected every 20 minutes until the raw materials disappeared completely, and then separated and extracted with ethyl acetate and saturated brine to obtain 2-(2-chlorophenyl)-2-oxoacetaldehyde.
[0096] Transfer the prepared 2-(2-chlorophenyl)-2-oxoacetaldehyde to a 50mL pear-shaped flask, add 5 mL of 1,2-dichloroethane, add isopropylamine (141.8mg), 25 Reaction at ℃ temperature for 2h. Sodium borohydride (90.8mg) was added to the system several times in small amounts, and reacted at 0°C for 2h. After the solvent was removed by rotary evaporation, it was separated by silica gel column (eluent, petroleum ether: ethyl acetate = 5:1 to 0:1) to obtain chlorprerenaline, 232 mg of white solid.
[0097] The melting point of the white solid was me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com