Magnesium-based hydrogen storage material capable of absorbing hydrogen at room temperature and preparation method thereof
A hydrogen storage material and room temperature technology, which is applied in the field of magnesium-based hydrogen storage materials absorbing hydrogen at room temperature and its preparation, can solve the problems of unfavorable practical application of magnesium-based hydrogen storage materials, achieve excellent catalytic activity, reduce hydrogen absorption and desorption temperature, Effect of improving hydrogen absorption and desorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
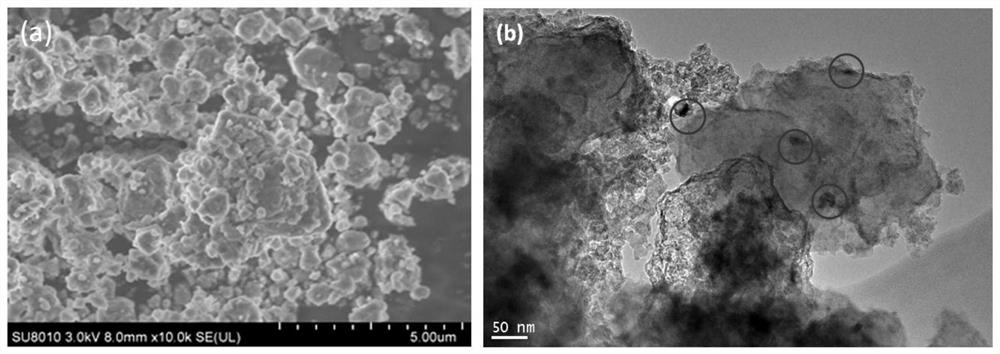
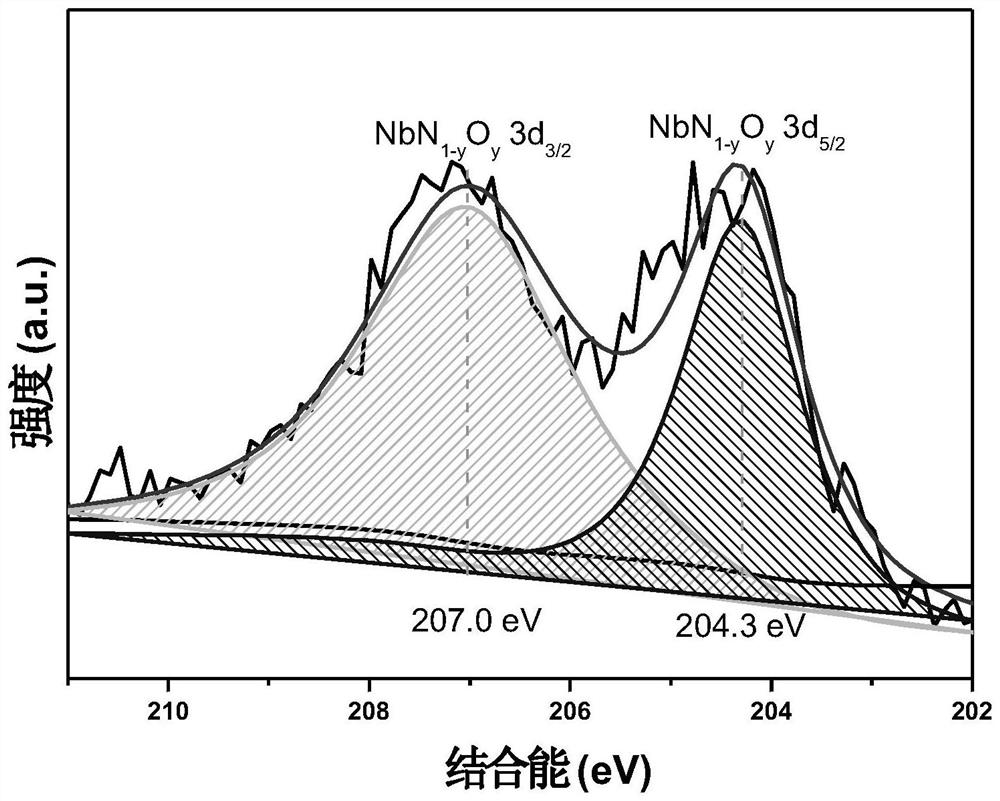
[0041] (1) N-doped niobium pentoxide (referred to as N-Nb 2 o 5 ) preparation: add 0.03mol niobium ethanol to 20mL benzyl alcohol, stir until pale yellow, add 0.06mol triethylamine (the molar ratio of triethylamine and niobium ethanol is 2:1), stir well and seal it into the reaction vessel, Insulated at 250°C for 3 days, the product was washed with ethanol and dried at 90°C to obtain an N-doped niobium oxide-based catalyst with a molar ratio of N element to Nb element of 0.06:1.
[0042] (2) Ball milling treatment: In a glove box with an argon atmosphere, MgH 2 and N-Nb 2 o 5 Mix with a mass ratio of 10:1, put it into a stainless steel ball mill tank with balls, and perform ball milling to obtain a mixture;
[0043] Wherein, the ball-to-material ratio is 120:1, and ball milling is carried out at room temperature for 24 hours at a rotational speed of 500 rpm.
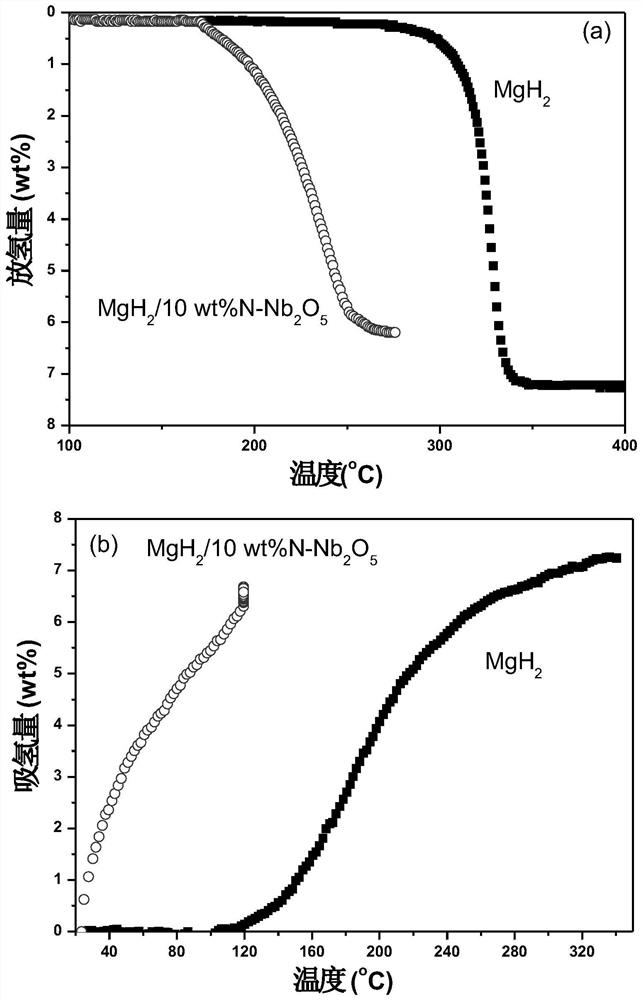
[0044] (3) Activation treatment: After ball milling, the mixture is first dehydrogenated at 300°C for 1 hour, and...
Embodiment 2
[0051] (1) N-doped niobium pentoxide (referred to as N-Nb 2 o 5 ) preparation method: add 0.03mol niobium ethanol to 20mL benzyl alcohol, stir until pale yellow, add 0.03mol triethylamine (the molar ratio of triethylamine and niobium ethanol is 1:1), stir well and seal it into the reaction kettle , kept at 250°C for 3 days, the product was washed with ethanol and dried at 90°C to obtain an N-doped niobium oxide-based catalyst with a molar ratio of N element to Nb element of 0.02:1.
[0052] (2) Ball milling treatment: In a glove box with an argon atmosphere, MgH 2 and N-Nb 2 o 5 Mix with a mass ratio of 100:1, put it into a stainless steel ball mill tank with balls, and perform ball milling to obtain a mixture;
[0053] Wherein, the ball-to-material ratio is 120:1, and ball milling is carried out at room temperature for 24 hours at a rotational speed of 500 rpm.
[0054] (3) Activation treatment: the mixture after ball milling is first dehydrogenated at 450°C for 2 hours,...
Embodiment 3
[0058] (1) N-doped TiNb 2 o 7 (referred to as N-TiNb 2 o 7 ) preparation method: 0.002mol niobium chloride and 0.001mol titanium tetraisopropoxide were added to 44mL ethanol, after stirring, 0.01mol triethylamine was added (the molar ratio of triethylamine and niobium chloride was 5:1), After stirring evenly, put it into a reaction kettle and keep it warm at 250°C for 3 days. The product was washed with ethanol and dried at 90°C to obtain an N-doped niobium oxide-based catalyst with a molar ratio of N element to Nb element of 0.12:1.
[0059] (2) Ball milling treatment: In a glove box with an argon atmosphere, MgH 2 and N-TiNb 2 o 7 Mix with a mass ratio of 10:1, put it into a stainless steel ball mill tank with balls, and perform ball milling to obtain a mixture;
[0060] Wherein, the ball-to-material ratio is 120:1, and ball milling is carried out at room temperature for 24 hours at a rotational speed of 500 rpm.
[0061] (3) Activation treatment: the mixture after ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com