Prodrug with anti-tumor activity and preparation and application thereof
A technology of anti-tumor activity and prodrug, which is applied in the direction of anti-tumor drugs, organic active ingredients, medical preparations of non-active ingredients, etc., to achieve the effects of improving drug efficacy, good tumor targeting, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The operation, deprotection and concentration determination process of embodiment 1 DNA synthesizer
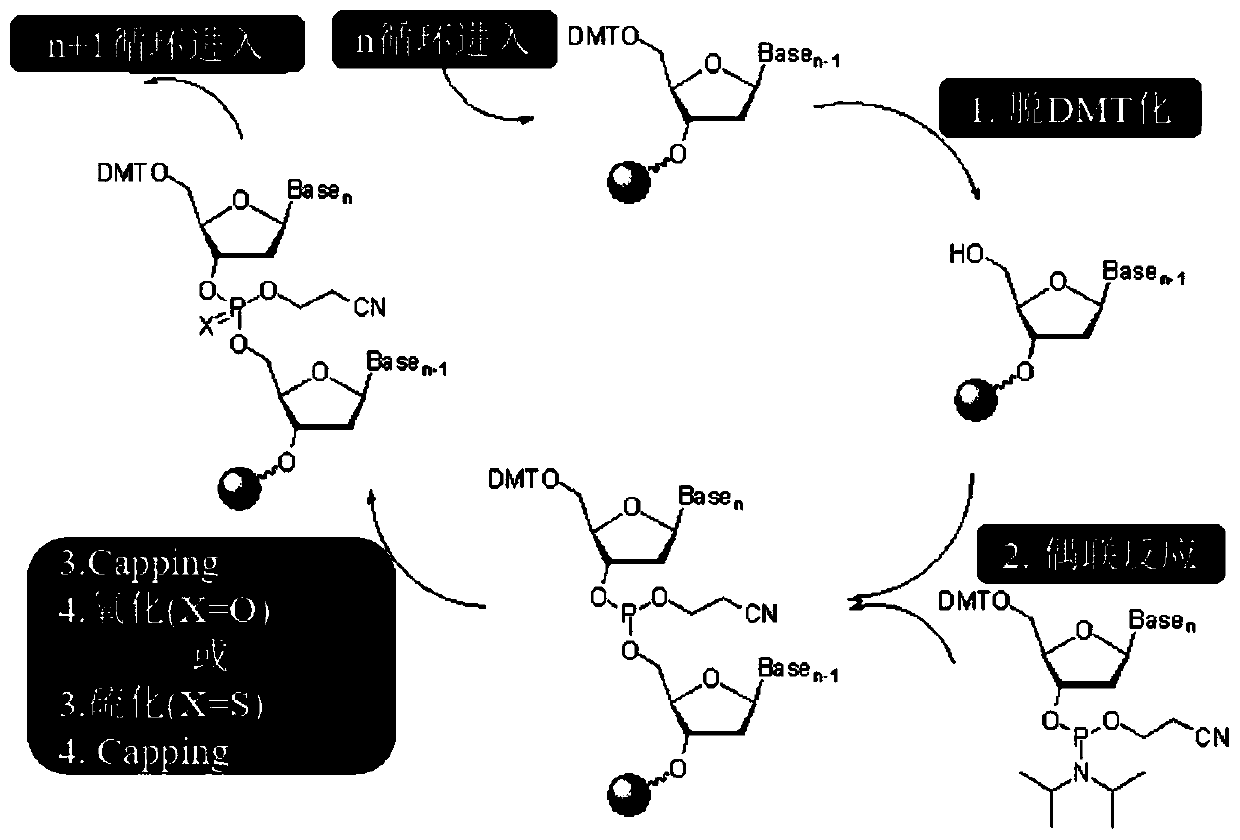
[0045] 1) Operation of DNA synthesizer: Taking the synthesis of phosphoramidite oligonucleotide as an example to introduce the operation steps of the DNA synthesis instrument produced by polygen ( figure 1 ):
[0046] (1) Preparation of synthetic reagents: 5'-DMT for protecting bases, A, G, C, T phosphoramidite monomers, tetrazole coupling catalysts, acetic anhydride, N-methylimidazole blocking reagents, trichloroacetic acid ( TCA) deprotection solution, oxidation mixture, acetonitrile cleaning solvent, ammonia removal solution, etc.
[0047] (2) Carefully check the amount of reagents in all reagent bottles on the synthesizer, and replace the reagent bottles if necessary, paying special attention to the amount of anhydrous acetonitrile, because the amount of this solvent is relatively large.
[0048] (3) Put the marked clean collection bottle on the synthesizer.
[...
Embodiment 2
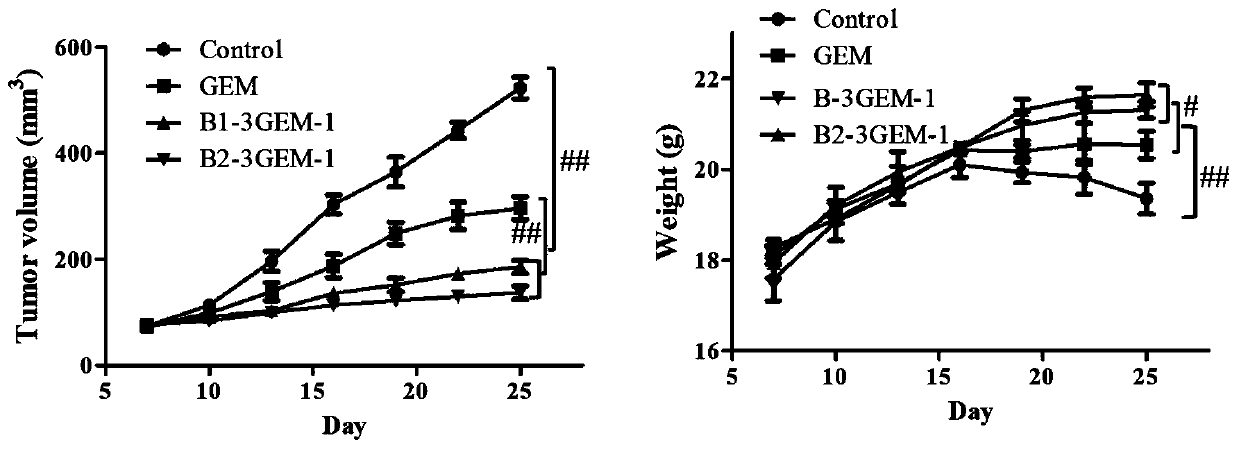
[0081] Embodiment 2 has the preparation of prodrug (B-3GEM-1) of antitumor activity
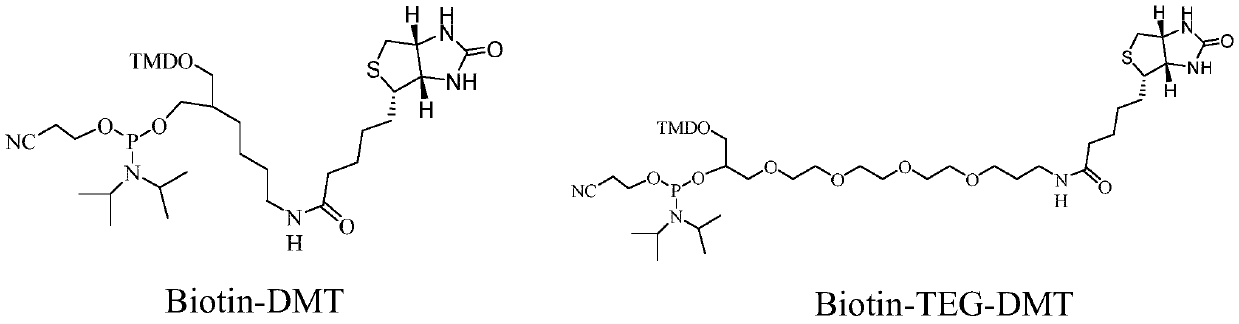
[0082]Put the phosphoramidite base monomer (A / T / C / G / GEM(Z) / Biotin(B)) into the corresponding anhydrous reagent bottle of the nucleic acid synthesizer, and design according to the standard procedure of the DNA synthesizer The sequence 5'-B-CTT ZZZ CCG GCG-3' is automatically synthesized. After the synthesis is completed, DMT cutting, desalting precipitation and HPLC separation and purification are carried out in sequence according to the standard procedure. The purity is greater than 95%, and the yield is not less than 75%. The mass spectrometry showed that the molecular weight was 4102.1, which was consistent with the calculated molecular weight of 4101.4, and 5'-B-CTT ZZZ CCG GCG-3' was obtained.
Embodiment 3
[0083] Embodiment 3 has the preparation of the prodrug (B-3GEM-2) of antitumor activity
[0084] Put the phosphoramidite base monomer (A / T / C / G / GEM(Z) / Biotin(B)) into the corresponding anhydrous reagent bottle of the nucleic acid synthesizer, and design according to the standard procedure of the DNA synthesizer The sequence 5'-B-TTT ZCZ CZG GCC-3' is automatically synthesized. After the synthesis is completed, DMT cutting, desalting precipitation and HPLC separation and purification are carried out in sequence according to the standard procedure. The purity is greater than 94%, and the yield is not less than 76%. The mass spectrometry showed that the molecular weight was 4102.0, which was consistent with the calculated molecular weight of 4101.4, and 5'-B-CTT ZCZ CZG GCC-3' was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com