A temperature and enzyme dual responsive protein polymer conjugate and its preparation method and application
A temperature-responsive and enzyme-responsive technology, which is applied in the direction of peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve high specificity and improve the effect of drug circulation half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
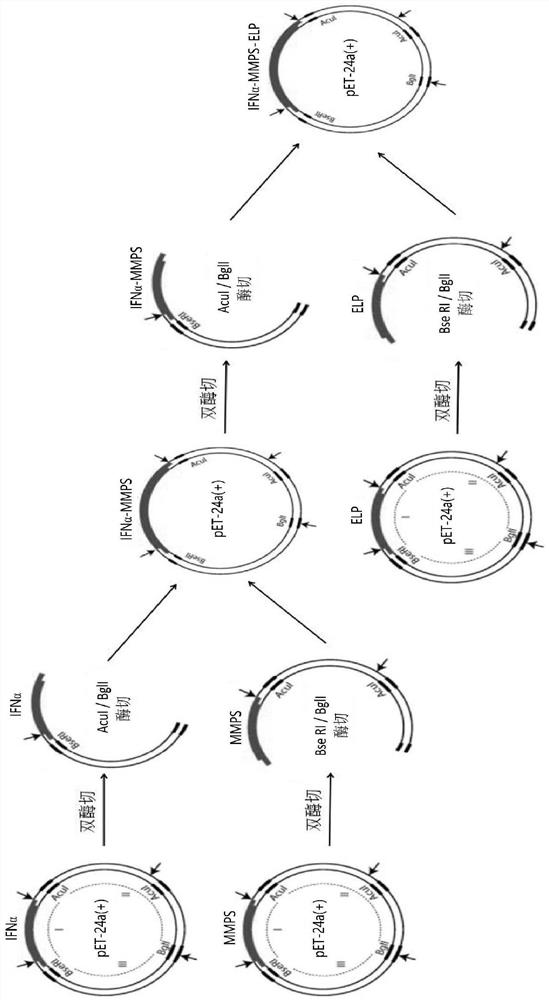
[0104] Example 1 IFNα-MMPS-ELP fusion protein plasmid construction and expression in Escherichia coli
[0105] The present invention obtains ELPs with different sequences and molecular sizes by designing and optimizing the amino acid sequence of the ELP repeating unit and the number of repetitions of the repeating unit, and determines the ELP with excellent performance through screening (both ensuring that the phase transition temperature is properly lower than body temperature, It is in the form of a gel in the body to achieve aggregation in the body and slow release of protein drugs into the blood, making the drug both long-acting and sustained release with good tissue permeability). In this embodiment, the amino acid sequence of the ELP repeat unit is VGVPG, and the repeat number n is 90, named ELP(V) 90 Taking the excellent performance of ELP as an example, ELP(V) 90 The phase transition temperature is in the range of 20-36 °C.
[0106] Gene fragments containing the abov...
Embodiment 2
[0123] Example 2 Purification of IFNα-MMPS-ELP and IFNα-ELP Fusion Protein
[0124] 1. Use Inverse transition cycling (ITC) to purify IFNα-MMPS-ELP and IFNα-ELP. The specific method is as follows:
[0125] (1) Collect 1L of Escherichia coli culture solution in a centrifuge bottle, collect the cells by centrifugation at 3000×g, and remove the supernatant culture solution.
[0126] (2) The cells were resuspended in 30 mL of ice-cold PBS, and the cells were disrupted by an ultrasonic instrument at 4° C., and then the crushed E. coli products were centrifuged at 4° C. and 14,000×g centrifugal force for 15 minutes.
[0127] (3) Add 2 mL of polyethyleneimine (PEI, 10%) to the supernatant collected in step (2), and centrifuge again for 15 minutes, in order to remove nucleic acid and other negatively charged substances in the cell lysate, and obtain the above ITC purification of the supernatant: add NaCl with a final concentration of 3M, fully dissolve at 37°C and centrifuge at 14,00...
Embodiment 3
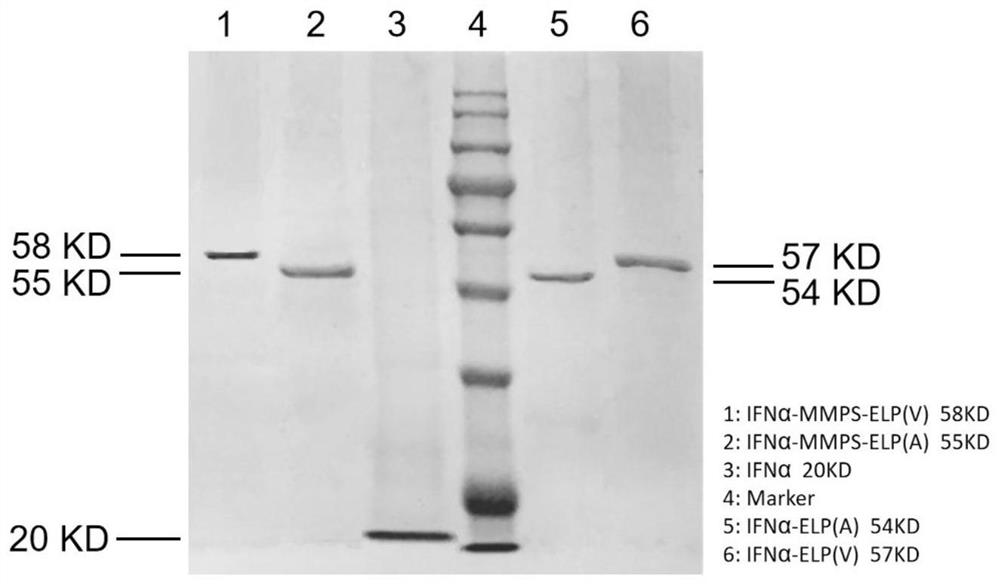
[0130] Example 3 Determination of Physicochemical Characterization Parameters of IFNα-MMPS-ELP and IFNα-ELP Fusion Proteins
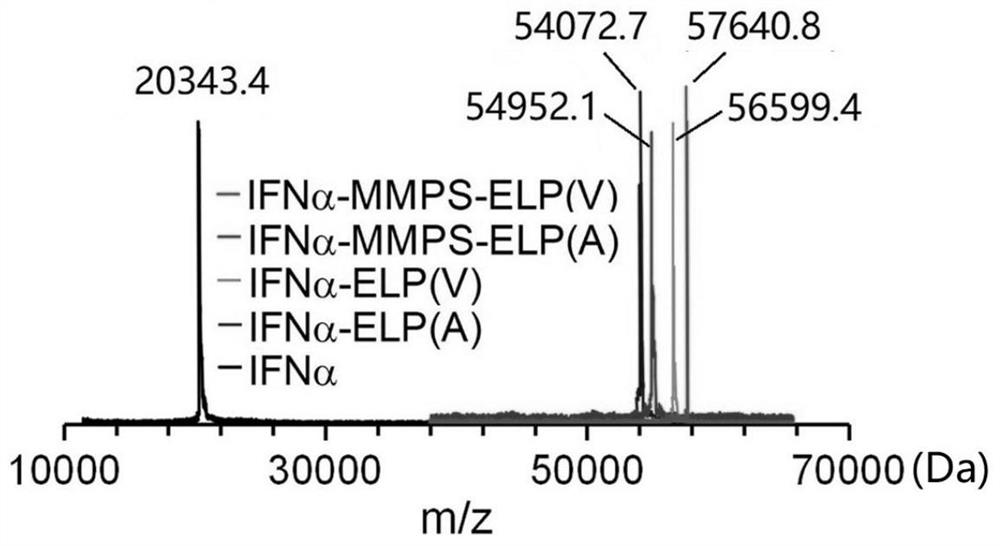
[0131] 1. Measure the molecular weight of the purified product obtained in Example 2 with matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF), and the instrument used is 4800PlusMALDI-TOF / TOF TM Analyzer (AB SCIEX), the results are as image 3 As shown, the experimentally measured molecular weights of IFNα-MMPS-ELP, IFNα-ELP and IFNα are close to the theoretical values.
[0132] 2. The hydration radius of the sample was determined by the dynamic light scattering (DLS) method on Malvern Zetasizer Nano-zs90: the sample was diluted in PBS buffer and filtered through a 0.22 μm pore size filter before testing. According to the DLS test, the hydration radius of IFNα is 2.9nm, while the hydration radius of the synthesized protein-polymer conjugate reaches 11nm. The kidney filtration clearance size is about 5nm radius, ind...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com