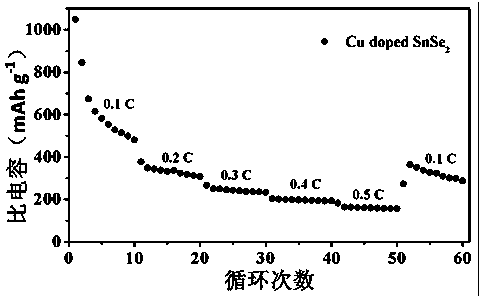
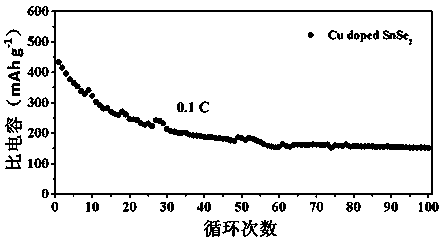
Hydrothermal method for preparing Cu-doped SnSe2 lithium ion battery electrode material
A lithium-ion battery and electrode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of easy cracking and pulverization of electrode materials, loss of electrical contact of active materials, deterioration of electrochemical performance, etc., and achieve excellent electrochemical performance. Performance, low cost, and the effect of improving discharge specific capacitance and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] According to the molar ratio (Sn:Se=1:2), weigh a certain amount of SnCl 2 and SeO 2 , put the two reaction materials into the liner of a 50 ml hydrothermal reaction kettle, and clean the liner with deionized water and absolute ethanol in advance. According to a certain mass ratio (SnCl 2 : Cu(NO 3 ) 2=5:1) Weigh a certain amount of copper nitrate solid particles and put them into the inner tank as well. Add a certain amount of deionized water to the inner tank to ensure that the filling volume is 80%, and then carry out magnetic stirring for 30 minutes, then put the inner tank into the reaction kettle and seal it. The reactor was placed in a constant temperature drying oven at 180 °C for 24 h and then cooled to room temperature naturally. The obtained product was washed with deionized water and absolute ethanol alternately for three times, and then dried in an incubator at 80 °C for 12 h.
Embodiment 2
[0034] According to the molar ratio (Sn:Se=1:2), weigh a certain amount of SnCl 2 and SeO 2 , put the two reaction materials into the liner of a 50 ml hydrothermal reaction kettle, and clean the liner with deionized water and absolute ethanol in advance. According to a certain mass ratio (SnCl 2 : Cu(NO 3 ) 2 =5:1) Weigh a certain amount of copper nitrate solid particles and put them into the inner tank as well. Add a certain amount of deionized water to the inner tank to ensure that the filling volume is 80%, and then carry out magnetic stirring for 30 minutes, then put the inner tank into the reaction kettle and seal it. The reactor was placed in a constant temperature drying oven at 160 °C for 24 h and then cooled to room temperature naturally. The obtained product was washed with deionized water and absolute ethanol alternately for three times, and then dried in an incubator at 80 °C for 12 h.
Embodiment 3
[0036] According to the molar ratio (Sn:Se=1:2), weigh a certain amount of SnCl 2 and SeO 2 , put the two reaction materials into the liner of a 50 ml hydrothermal reaction kettle, and clean the liner with deionized water and absolute ethanol in advance. According to a certain mass ratio (SnCl 2 : Cu(NO 3 ) 2 =5:1) Weigh a certain amount of copper nitrate solid particles and put them into the inner tank as well. Add a certain amount of deionized water to the inner tank to ensure that the filling volume is 80%, and then carry out magnetic stirring for 30 minutes, then put the inner tank into the reaction kettle and seal it. The reactor was placed in a constant temperature drying oven at 200 °C for 24 h and then cooled to room temperature naturally. The obtained product was washed with deionized water and absolute ethanol by alternating centrifugation for three times, and then dried in an incubator at 80°C for 12 h.
[0037] The chemical raw material SnCl used in the above...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com