A kind of phentolamine mesylate injection and preparation method thereof
A technology of phentolamine mesylate and injection, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of alkali damage and oxidative damage instability, and product stability It has the advantages of good effect, low production cost, and prevention of oxidative damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
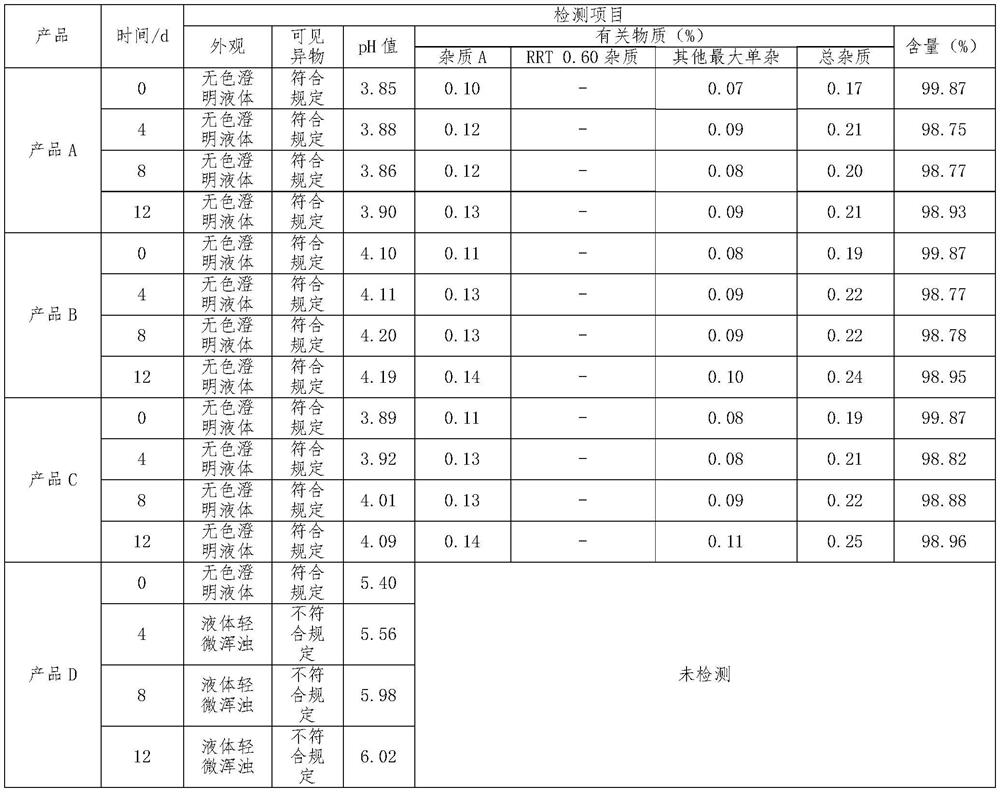
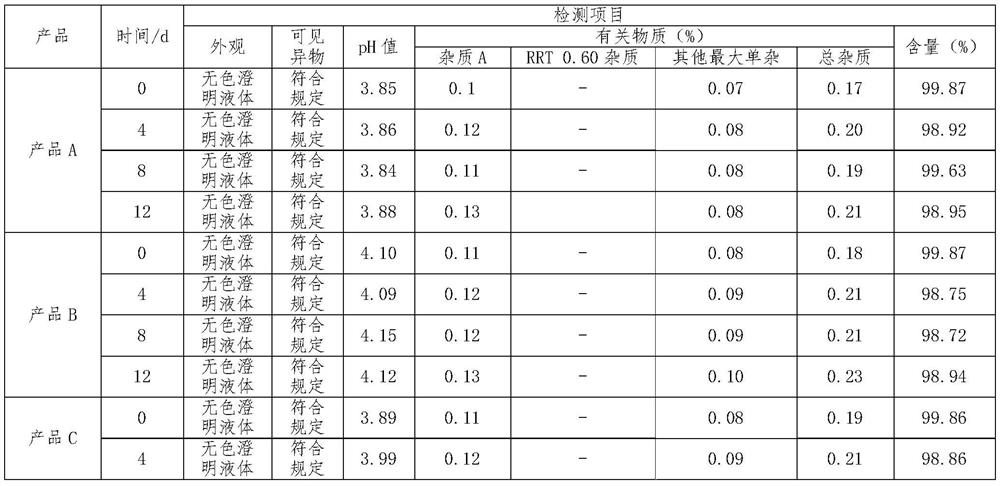
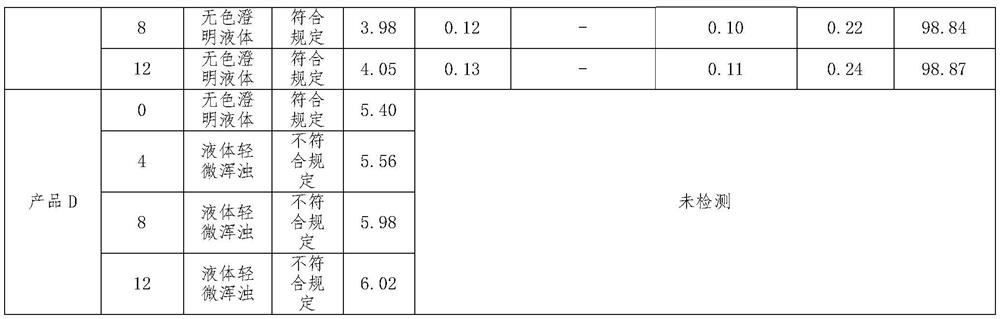
[0025] Raw materials: 16.32g of phentolamine mesylate, 0.68g of sodium metabisulfite, 3400g of mannitol, add 100ml-120ml of 3.6% dilute acetic acid, adjust the pH value to 3.8-4.0, add water for injection to 68L, and make 40000 sticks, each The size of the bottle is 1.7ml.
[0026] Preparation:
[0027] (1) medicinal solution configuration: a. take by weighing sodium metabisulfite and mannitol of prescription quantity, and dissolve it in the water for injection of 70% of prescription quantity; b. add the phentolamine mesylate of prescription quantity; c. Stir until fully dissolved; d. add water for injection to the prescribed amount; e. adjust the pH value to 3.8-4.0 with 3.6% dilute acetic acid solution; f. feed nitrogen into the prepared medicinal solution;
[0028] (2) Sterilization filling: a. Washing bottle: adopt neutral borosilicate glass ampoule, carry out the mode of dry heat sterilization in the tunnel oven after washing with water for injection, sterilization tempe...
Embodiment 2
[0031] Raw materials: 16.32g of phentolamine mesylate, 0.34g of sodium metabisulfite, 2040g of mannitol, add 2% dilute acetic acid, adjust the pH value to 4.0-4.2, add water for injection to 68L, and make 40,000 sticks, each with a specification of 1.7 ml.
[0032] Preparation:
[0033] (1) liquid medicine configuration: a. take by weighing sodium metabisulfite and mannitol of prescription quantity, and dissolve it in the water for injection of 65% of prescription quantity; b. add the phentolamine mesylate of prescription quantity; c. Stir until fully dissolved; d. add water for injection to the prescribed amount; e. adjust the pH value to 4.0-4.2 with 2% dilute acetic acid solution; f. feed nitrogen into the prepared medicinal solution;
[0034] (2) Sterilization filling: a. Washing bottle: adopt neutral borosilicate glass ampoule, carry out the mode of dry heat sterilization in the tunnel oven after washing with water for injection, sterilization temperature is 300-320 ℃, m...
Embodiment 3
[0037]Raw materials: 16.32g of phentolamine mesylate, 1.02g of sodium metabisulfite, 5440g of mannitol, add 5% dilute acetic acid, adjust the pH value to 3.8-4.2, add water for injection to 68L, and make 40,000 sticks, each with a specification of 1.7 ml.
[0038] Preparation:
[0039] (1) liquid medicine configuration: a. take by weighing sodium metabisulfite and mannitol of prescription quantity, and dissolve it in the water for injection of 60% of prescription quantity; b. add the phentolamine mesylate of prescription quantity; c. Stir until fully dissolved; d. add water for injection to the prescribed amount; e. adjust the pH value to 3.8-4.2 with 5% dilute acetic acid solution; f. feed nitrogen into the prepared medicinal solution;
[0040] (2) Sterilization filling: a. Washing bottle: adopt neutral borosilicate glass ampoule, carry out the mode of dry heat sterilization in the tunnel oven after washing with water for injection, sterilization temperature is 300-320 ℃, ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com