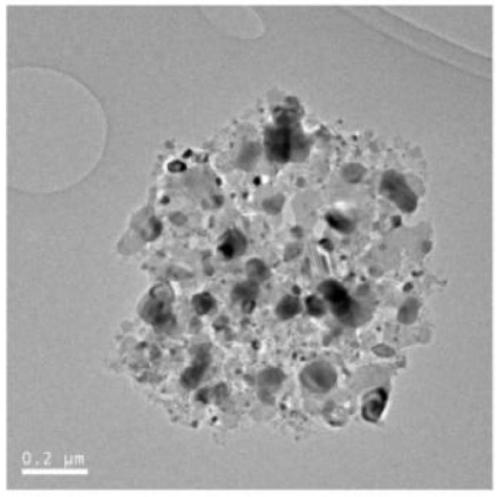
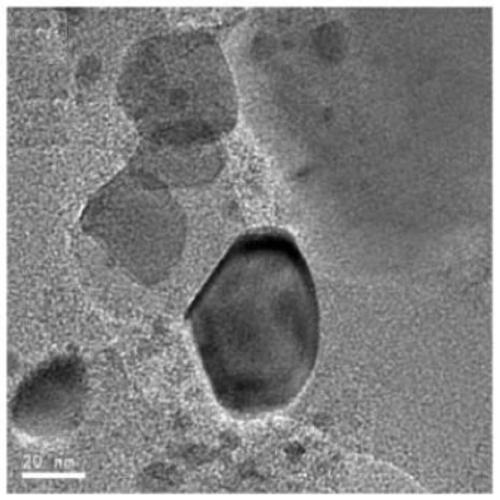
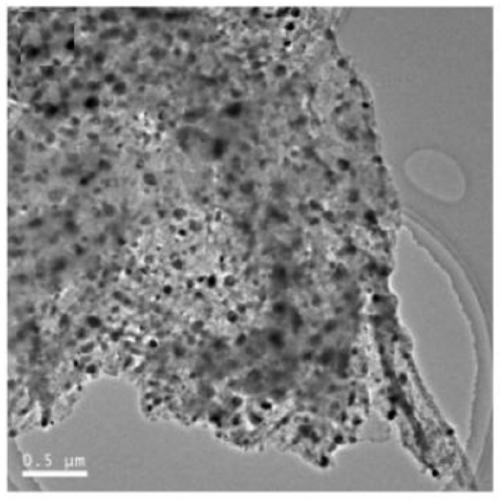
Carbon-based metal phosphide loaded catalyst, preparation method and application thereof
A technology of supporting metals and phosphides, applied in the field of electrocatalysis, can solve the problems of poor stability of pure Pt, reduced reactivity, and limited wide application, and achieve the effects of easy regulation, weakening adsorption, and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: RhP 2 Synthesis of @NC
[0041] 1) Add 50 mg of rhodium nitrate and 50 mL of deionized water into a 250 mL beaker, stir to dissolve, add 5 mL of phosphoric acid dropwise while stirring continuously, and stir for 40 minutes to form a uniform aqueous solution;
[0042] 2) Add 1 g of cyanamide to the aqueous solution obtained in step 1), and stir for 75 minutes, so that the cyanamide can be more evenly dispersed in the aqueous solution to obtain a precursor solution;
[0043] 3) Put the precursor solution obtained in step 2) into a polytetrafluoroethylene tank, conduct a hydrothermal reaction at 150°C for 20 hours, cool to room temperature after the reaction, filter the reaction solution, and vacuum-dry the obtained solid at 80°C 20 hours, obtain the rhodium phosphide precursor;
[0044] 4) Grind the rhodium phosphide precursor obtained in step 3) into a powder, weigh 1g of the rhodium phosphide precursor powder and transfer it to a tube furnace, pass nitrogen...
Embodiment 2
[0050] Example 2: IrP 2 Synthesis of @NC
[0051] 1) Add 100 mg of iridium tetrachloride and 50 mL of deionized water into a 250 mL beaker, stir to dissolve, add 10 mL of 50% phytic acid dropwise while stirring continuously, and stir for 30 minutes to form a uniform aqueous solution;
[0052] 2) Add 2 g of melamine to the aqueous solution obtained in step 1), and stir for 60 minutes, so that the melamine can be more evenly dispersed in the aqueous solution to obtain a precursor solution;
[0053] 3) Put the precursor solution obtained in step 2) into a polytetrafluoroethylene tank, conduct a hydrothermal reaction at 120°C for 24 hours, cool to room temperature after the reaction, filter the reaction solution, and vacuum-dry the obtained solid at 60°C After 25 hours, the iridium phosphide precursor was obtained;
[0054] 4) Grind the iridium phosphide precursor obtained in step 3) into a powder, weigh 1g of the iridium phosphide precursor powder and transfer it to a tube furn...
Embodiment 3
[0059] Example 3: Pd 5 P 2 Synthesis of @NC
[0060] 1) Add 75 mg of palladium chloride and 70 mL of deionized water into a 250 mL beaker, stir to dissolve, add 5 g of sodium dihydrogen phosphate dropwise while stirring continuously, and stir for 45 minutes to form a uniform aqueous solution;
[0061] 2) Add 3 g of urea to the aqueous solution obtained in step 1), and stir for 100 minutes, so that the urea can be more evenly dispersed in the aqueous solution to obtain a precursor solution;
[0062] 3) Put the precursor solution obtained in step 2) into a polytetrafluoroethylene tank, conduct a hydrothermal reaction at 180°C for 10 hours, cool to room temperature after the reaction, filter the reaction solution, and vacuum-dry the obtained solid at 100°C 20 hours, obtain palladium phosphide precursor;
[0063] 4) Grind the palladium phosphide precursor obtained in step 3) into a powder, weigh 1g of the palladium phosphide precursor powder and transfer it to a tube furnace, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com