Hemostatic material for nephrology department and preparation method thereof
A hemostatic material and internal medicine technology, applied in the field of medical materials, can solve the hidden dangers of fibrin glue virus, tissue necrosis and other problems, achieve good hemostatic effect, promote the formation of thrombus, and produce low cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
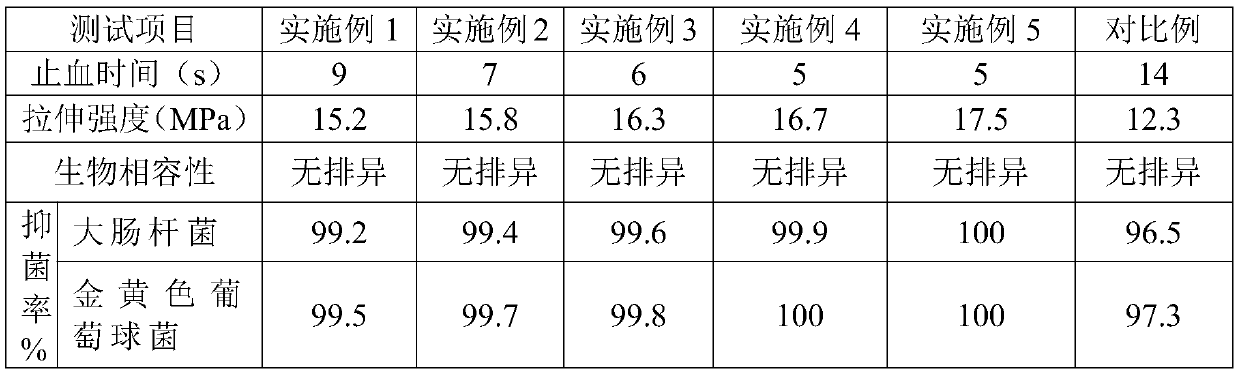
Embodiment 1
[0034] A preparation method of a hemostatic material for nephrology, comprising the steps of:
[0035]Step S1 Ionization of epichlorohydrin bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl]ethylamino]adipate: 100g of epichlorohydrin 335g of bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl]ethylamino]adipate was added to 1200g of dichloromethane and stirred at 30°C After reacting for 6 hours, the dichloromethane was removed by rotary evaporation, and the product was washed 3 times with ether, and then the ether was removed by rotary evaporation to obtain the ionized di[2-[[4-(2,2-dicyanovinyl )-3-methylphenyl]ethylamino]adipate;
[0036] Preparation of step S2 modified cyclodextrin: ionizing epichlorohydrin prepared in step S1 to bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl] Add 100g of ethylamino]adipate, 300g of mono-6-O-amino-β-cyclodextrin, and 10g of chlorobenzoquinone into 2000g of N,N-dimethylformamide, and stir the reaction at 60°C for 6 hours, then precipitated in acetone, rem...
Embodiment 2
[0044] A preparation method of a hemostatic material for nephrology, comprising the steps of:
[0045] Step S1 Ionization of epichlorohydrin bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl]ethylamino]adipate: 100g of epichlorohydrin 335g of bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl]ethylamino]adipate was added to 1350g of ethyl acetate and stirred at 32°C After reacting for 6.5 hours, the ethyl acetate was removed by rotary evaporation, and the product was washed 4 times with ether, and then the ether was removed by rotary evaporation to obtain epichlorohydrin ionized bis[2-[[4-(2,2-dicyanovinyl )-3-methylphenyl]ethylamino]adipate;
[0046] Preparation of step S2 modified cyclodextrin: ionizing epichlorohydrin prepared in step S1 to bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl] Add 100g of ethylamino]adipate, 350g of mono-6-O-amino-β-cyclodextrin, and 15g of l,4-naphthoquinone into 2100g of N,N-dimethylformamide, and stir at 65°C Reacted for 6.5 hours, then precipitated in ace...
Embodiment 3
[0054] A preparation method of a hemostatic material for nephrology, comprising the steps of:
[0055] Step S1 Ionization of epichlorohydrin bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl]ethylamino]adipate: 100g of epichlorohydrin 335 g of bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl]ethylamino]adipate was added to 1500 g of acetone, and the reaction was stirred at 35°C for 7 hours, then remove the acetone by rotary evaporation, and wash the product with ether for 3-5 times, then remove the ether by rotary evaporation to obtain the ionized bis[2-[[4-(2,2-dicyanovinyl)- 3-Methylphenyl]ethylamino]adipate;
[0056] Preparation of step S2 modified cyclodextrin: ionizing epichlorohydrin prepared in step S1 to bis[2-[[4-(2,2-dicyanovinyl)-3-methylphenyl] Add 100g of ethylamino]adipate, 400g of mono-6-O-amino-β-cyclodextrin, and 20g of chlorobenzoquinone into 2300g of N,N-dimethylformamide, and stir the reaction at 70°C for 7 hours, then precipitated in acetone, removed the acetone b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com