The synthetic method of middle ring lactam compound
A technology for amide compounds and lactams, which is applied in the field of synthesis of mesocyclic lactams, can solve the problem that the synthesis method of mesocyclic lactams has not been reported in domestic and foreign literatures, the difficulty of drug purification is increased, the experimental operation is dangerous, etc. problems, to achieve the effect of clean and environmentally friendly reaction system, green reaction system, and novel reaction mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
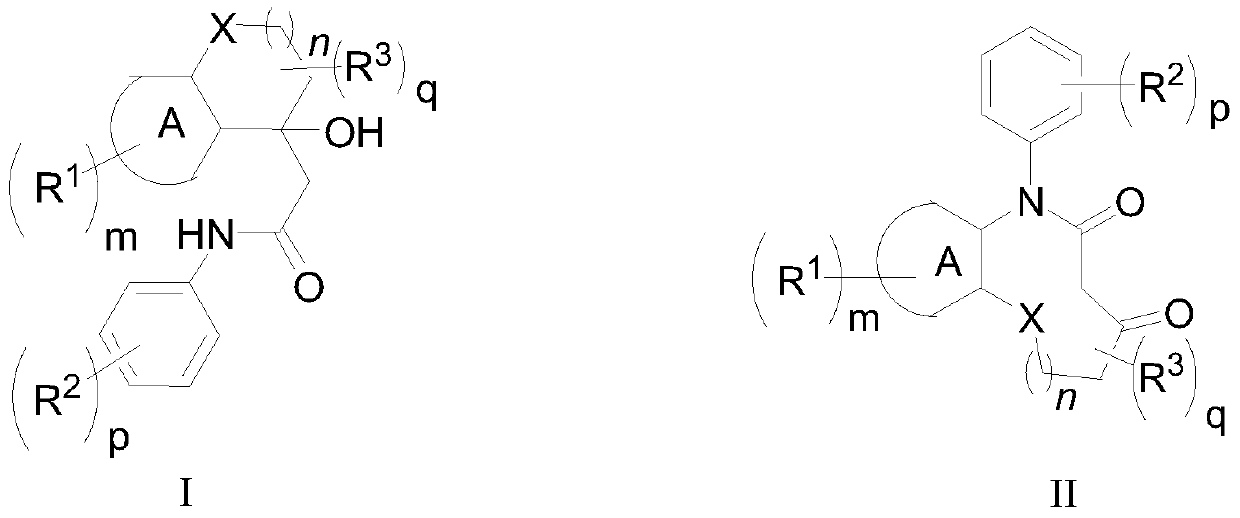
[0056] The invention provides a kind of synthetic method of middle lactam compound, comprises the steps:
[0057] Electrolyzing the amide compound with the structure shown in formula (I) to obtain the mesocyclic lactam compound with the structure shown in formula (II);
[0058]
[0059] in:
[0060] Ring A is: benzene ring, or 5-6 membered heteroaromatic ring;
[0061] R 1 For: H, halogen, C 1 ~C 20 Alkyl, C 6 ~C 20 Aryl, or C 1 ~C 20 alkoxy;
[0062] R 2 For: H, halogen, C 1 ~C 20 Alkyl, halogen substituted C 1 ~C 20 Alkyl, C 6 ~C 20 Aryl, C 1 ~C 20 Alkoxy, or two adjacent R 2 Together with the C atom it is attached to form C 6 ~C 10 Aryl;
[0063] R 3 For: H, halogen, C 1 ~C 20 Alkyl, C 6 ~C 20 Aryl, C 1 ~C 20 Alkoxy, or two adjacent R 3 Together with the C atom it is attached to form C 6 ~C 10 Aryl;
[0064] -X- is: O, S, or CR 3 ;
[0065] n is: 0, 1, 2 or 3;
[0066] m is: 0, 1, 2 or 3;
[0067] p and q are each independently: 0, 1, ...
Embodiment 1
[0088] Embodiment 1: Electrochemical method synthesis compound 2a
[0089]
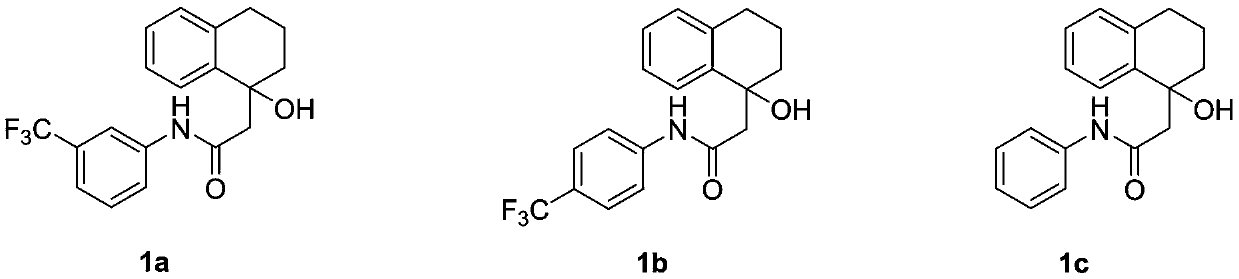
[0090] In a 10mL single-chamber electrolytic cell, the raw material 1a (0.2mmol) and the electrolyte Bu 4 NBF 4 (0.2mmol) was added in the mixed solvent system of 5mL acetonitrile and water (volume ratio is 9:1), with graphite sheet electrode as anode, platinum sheet as cathode, under the condition of 8mA constant electric current, electrolyze, stir at room temperature 2.3 hours ( The electric quantity is 3.4 F / mol based on the material amount of the raw material 1a), and then the electrolysis is stopped, the reaction solution is transferred, concentrated, separated and purified by column chromatography to obtain 2a as a white solid, and the yield is 98%.
[0091] The characterization data of compound 2a are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.74(s,1H),7.59–7.51(m,1H),7.51–7.39(m,4H),7.33–7.24(m,2H),3.43(s,2H),2.98–2.82(m, 1H),2.78–2.68(m,1H),2.60(td,J=13.1,2.7Hz,1H),2.39–2.04(m,3H);
[00...
Embodiment 2
[0095] Embodiment 2: electrochemical method synthesis compound 2b
[0096]
[0097] In a 10mL single-chamber electrolytic cell, the raw material 1b (0.2mmol) and the electrolyte Bu 4 NBF 4 (0.2mmol) was added in the mixed solvent system of 5mL acetonitrile and water (volume ratio is 9:1), with graphite sheet electrode as anode, platinum sheet as cathode, under the condition of 8mA constant electric current, electrolyze, stir at room temperature 2.3 hours ( The electric quantity is 3.4 F / mol based on the material amount of the raw material 1b), and then the electrolysis is stopped, the reaction solution is transferred, concentrated, separated and purified by column chromatography to obtain 2b as a white solid, and the yield is 88%.
[0098] The characterization data of compound 2b are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.60(d, J=8.1Hz, 2H), 7.53(d, J=8.1Hz, 2H), 7.51–7.42(m, 2H), 7.32(d, J=7.5Hz, 1H), 7.28( d, J=7.5Hz, 1H), 3.43(s, 2H), 2.91–2.86(m, 1H), 2.71–2.68(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com