Escherichia coli VO outer membrane protein nanoparticle vaccine modified plga with chitosan and its preparation method and application
A technology of Escherichia coli and outer membrane protein, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the lack of functional groups that can be covalently modified on the surface of nanoparticles, Short residence time, limited application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
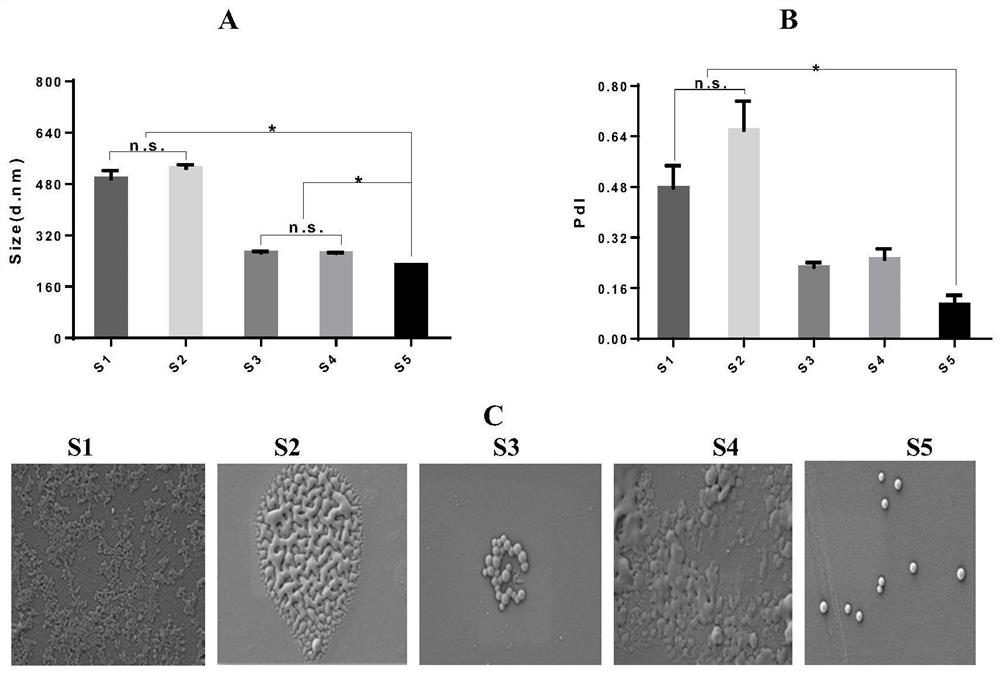
[0036] Embodiment 1, the preparation of different prescription nanoparticles
[0037] In order to determine the optimal formulation of nanoparticles, five groups of schemes were designed in this project: CS nanoparticles without PLGA (S1); PLGA nanoparticles with dichloromethane as solvent (S2); PLGA nanoparticles with acetone as solvent. particles (S3); PLGA-modified CS nanoparticles (S4); CS-modified PLGA nanoparticles (S5). The particle size, dispersion and three-dimensional shape of nanoparticles are detected by nanometer particle size analyzer and scanning electron microscope, and the optimal formula is obtained by screening.
[0038] Recipe 1: CS nanoparticles without PLGA (S1)
[0039] 1) Weigh 0.02g of chitosan (CS), add 20ml of glacial acetic acid with a volume fraction of 1%, and stir until completely dissolved to prepare a chitosan solution.
[0040] 2) Weigh 5 mg of sodium polyphosphate, add it into 5 ml of pure water, stir until completely dissolved, and prepare...
Embodiment 2
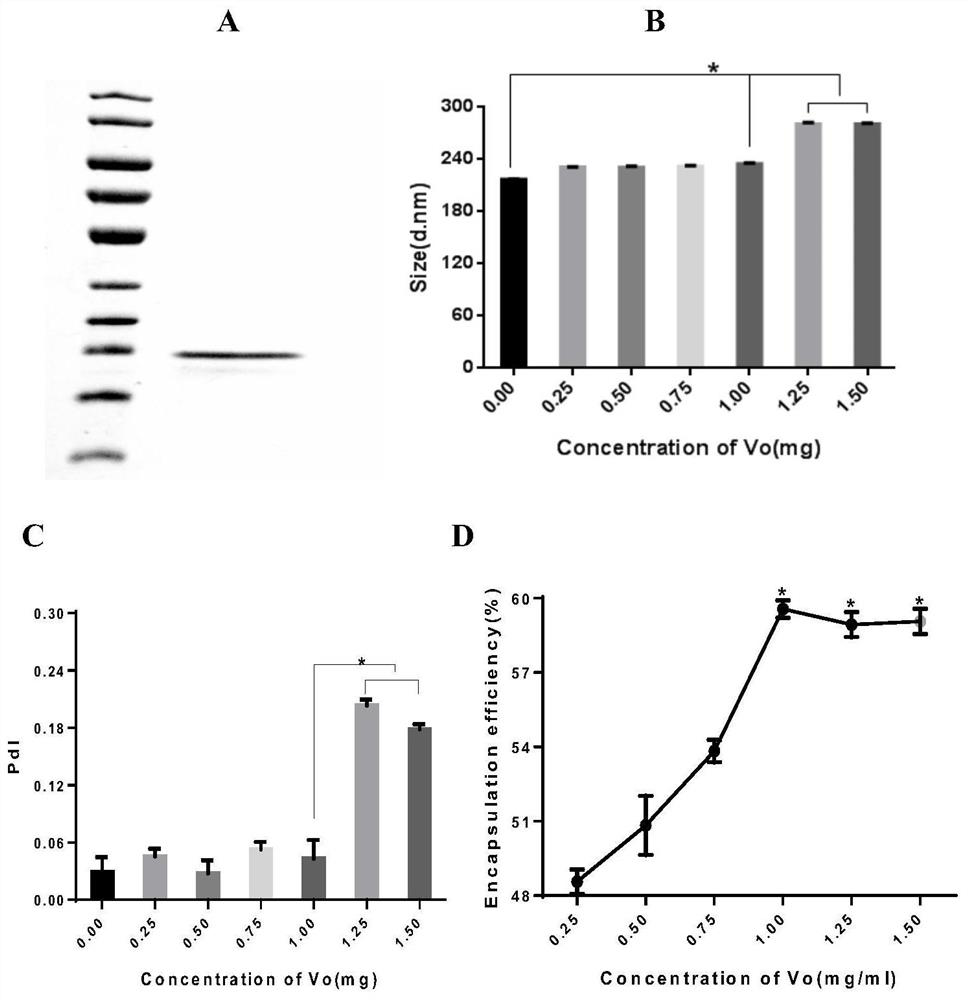
[0086] Embodiment 2, the determination of the optimum wrapping amount of Vo protein
[0087] In order to clarify the maximum Vo protein encapsulation capacity of this nanoparticle preparation process, the protein Vo was first purified and diluted to different concentrations (0.25mg / ml, 0.50mg / ml, 0.75mg / ml, 1.0mg / ml, 1.25 mg / ml, 1.5mg / ml), and prepared CS-PLGA nanoparticles wrapped with different concentrations of Vo protein. The particle size and PdI were analyzed by a nanometer particle size potentiometer, and the effect of loading different concentrations of Vo protein on the particle size and PDI value of nanoparticles was evaluated to determine the optimal wrapping amount of CS-PLGA nanoparticles. The specific method is as follows:
[0088] (1) Purification of Vo protein
[0089] 1) Take out the pGEX-Vo / X-blue strain (Gu, H.; Liao, Y.; Zhang, J.; Wang, Y.; Liu, Z.; Cheng, P.; Wang , X.; Zou, Q.; Gu, J., Rational Design and Evaluation of an Artificial Escherichia coli K1Pr...
Embodiment 3
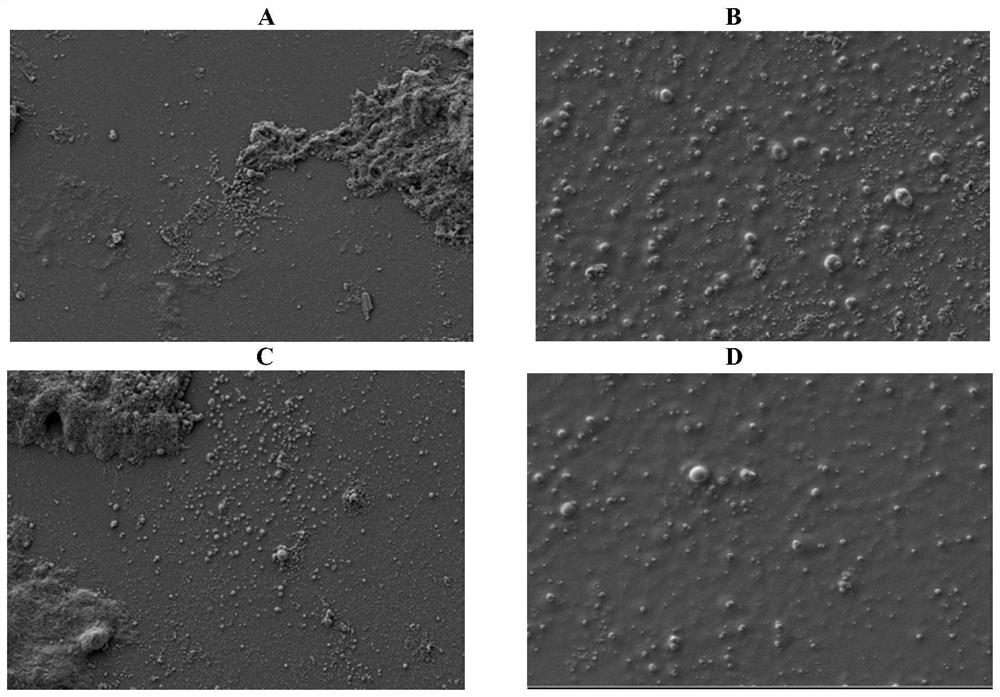
[0119] Embodiment 3, optimization of nanoparticle preparation process
[0120] In order to further improve the stability of VoNP, this study attempted to optimize the preparation process of nanoparticles by freeze-drying.
[0121] (1) Nanoparticles made by non-freeze-drying process
[0122] 1) Weigh 0.05g PLGA, add 5ml acetone, stir until completely dissolved.
[0123] 2) Weigh 0.02g CS, add it into 20ml 1% glacial acetic acid, stir until completely dissolved.
[0124] 3) Weigh 0.4g of PVA and 0.04g of sodium polyphosphate, add them into 15ml of pure water, and stir well.
[0125] 4) Add Vo protein 1.0mg / 5ml to the liquid in 3) (the blank control group only adds 5ml pure water).
[0126] 5) Under magnetic stirring at 800 rpm, add the liquid in 1) dropwise to 3).
[0127] 6) Under magnetic stirring at 800 rpm, add the liquid in 2) to 5) dropwise.
[0128] 7) Stir at 800 r for 8 hours in a fume hood (room temperature, wind power 60%).
[0129] 8) The sample is filtered wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com