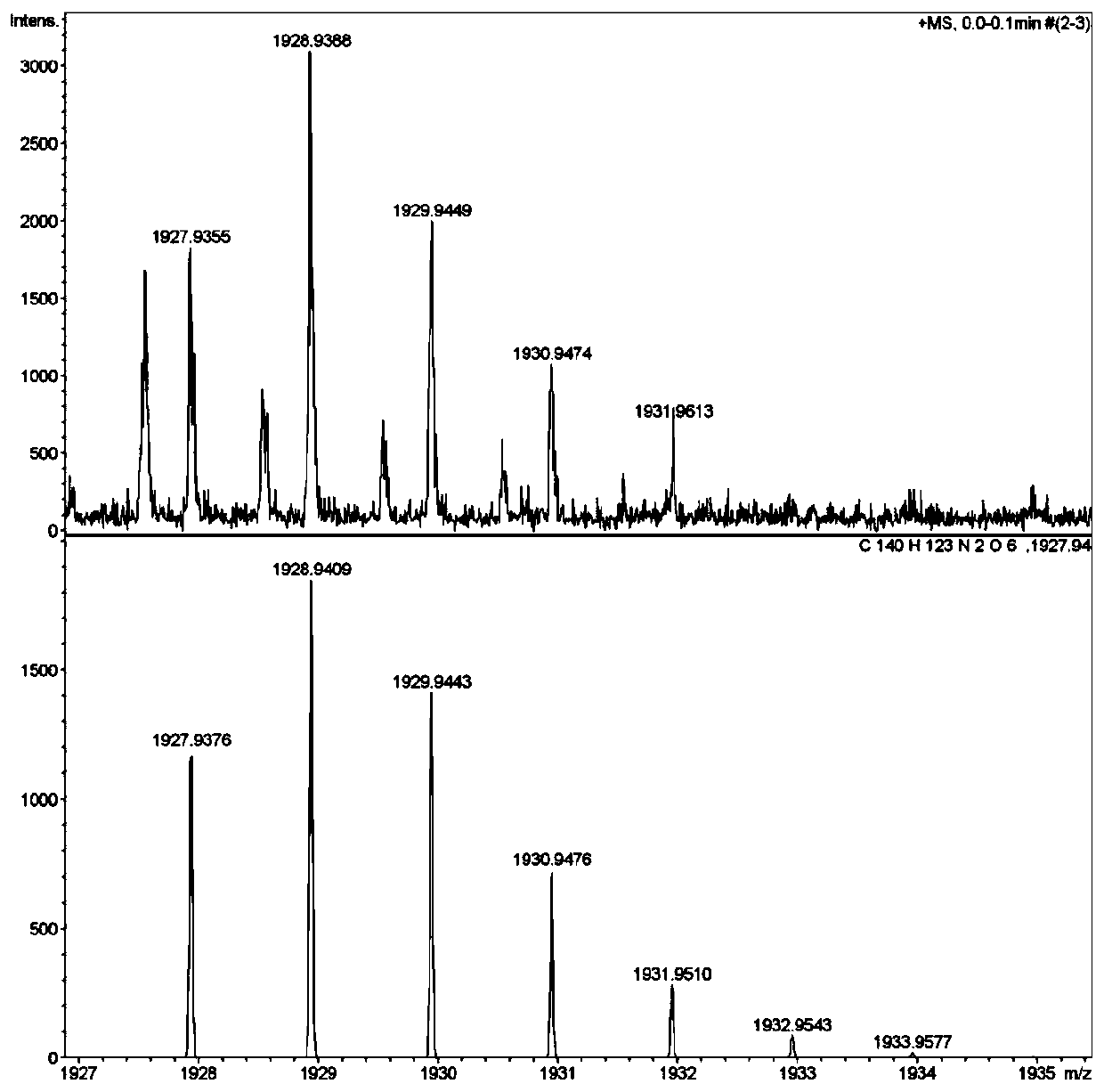
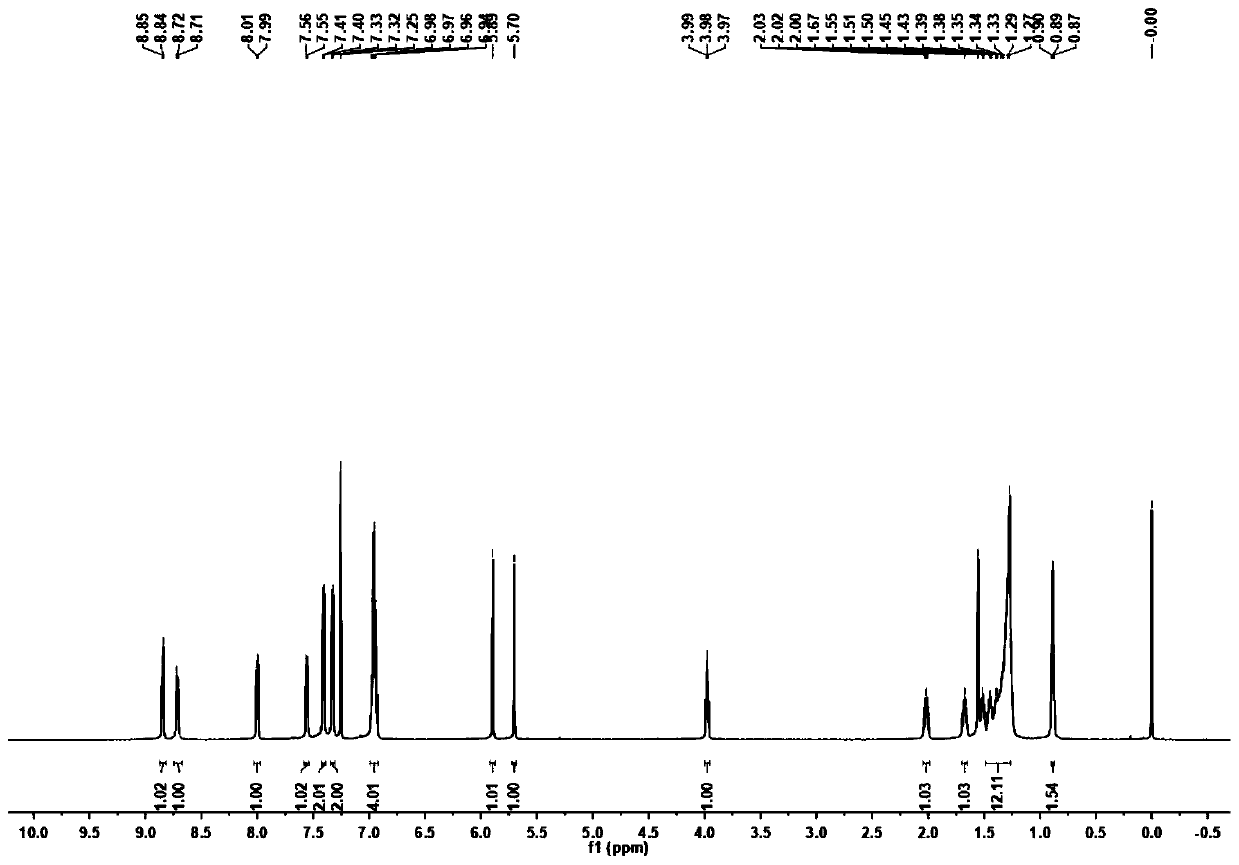
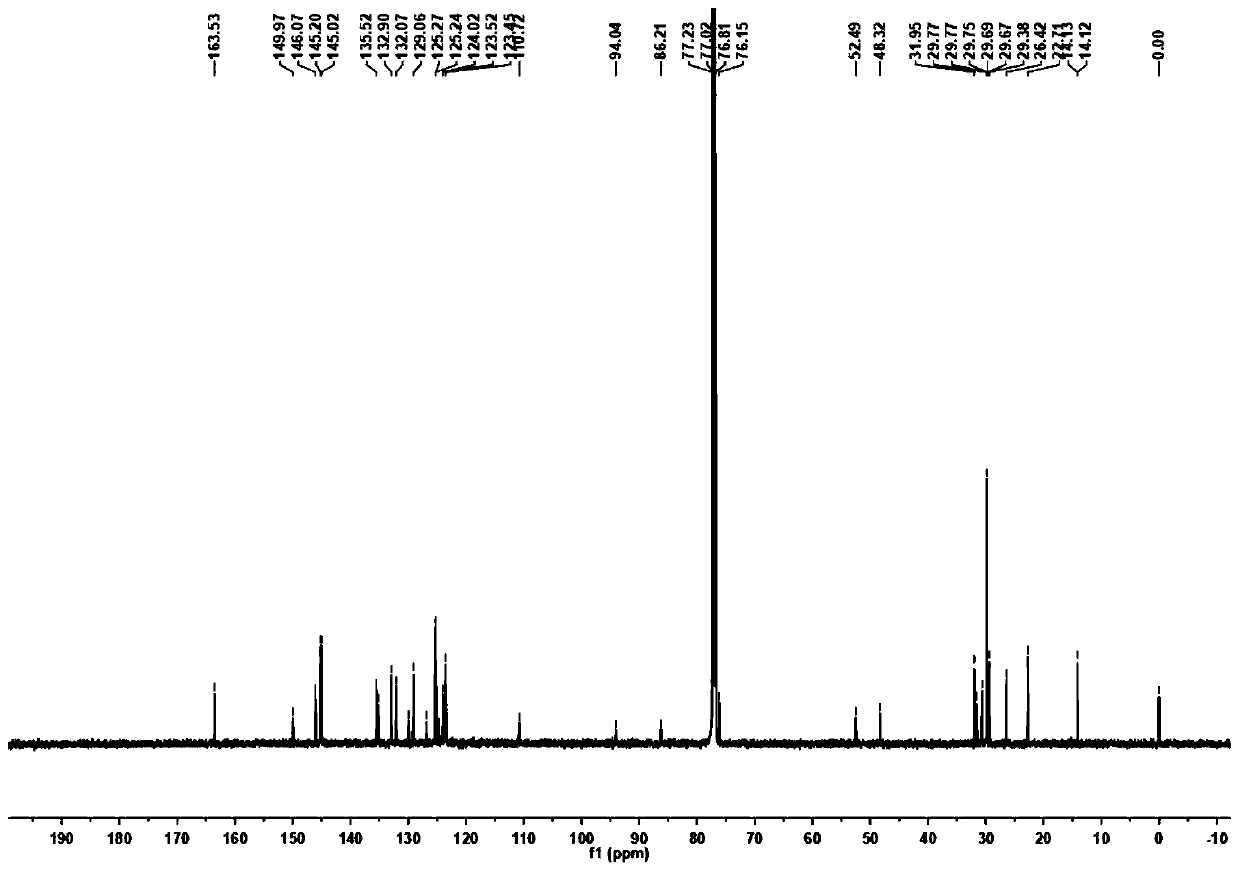
Ptycene-perylene diimide derivative, synthesis method thereof and sensing application to gas-phase volatile aromatic hydrocarbon
A technology of perylene diimide and synthesis method, which is applied in the field of small molecule fluorescence sensing thin film materials, can solve the problems of affecting the diffusion of analyte molecules, discount of thin film sensing performance, poor permeability of thin films, etc. The effect of aggregation-induced fluorescence quenching, fully reversible sensing process, and fast response and recovery times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of target fluorescent compound (p-iodoaniline is used in this example, alkyl chain n=16)
[0072] 1) Synthesis of compound 1a
[0073] Add 1.00g of perylenetetracarboxylic anhydride, 11.2g of p-iodoaniline, and 17g of imidazole into the flask in turn, and under an argon atmosphere, heat to 140°C under strong reflux and stir for 33 hours, then cool the reaction solution to room temperature naturally, add dichloromethane, and then The resulting suspension was filtered with suction, rinsed with methanol and chloroform in order to remove excess p-iodoaniline, and the obtained material was vacuum-dried to obtain brown-red compound 1a;
[0074] Its reaction equation is as follows:
[0075]
[0076] 2) Preparation of compound 2
[0077] Add 17.8g of anthracene, 5.4g of benzoquinone, and 75mL of mesitylene into a 200mL flask in sequence, reflux and stir for 24 hours, cool to room temperature, and filter. The obtained solid was added into 100 mL of hot xylene, ...
Embodiment 2
[0094] Preparation of target fluorescent compound (p-iodoaniline is used in this example, alkyl chain n=2)
[0095] 1) Synthesis of compound 1a
[0096]Add 1.00g of perylenetetracarboxylic anhydride, 11.2g of p-iodoaniline, and 17g of imidazole into the flask in turn, and under an argon atmosphere, heat to 140°C under strong reflux and stir for 33 hours, then cool the reaction solution to room temperature naturally, add dichloromethane, and then The resulting suspension was filtered with suction, rinsed with methanol and chloroform in order to remove excess p-iodoaniline, and the obtained material was vacuum-dried to obtain brown-red compound 1a;
[0097]
[0098] 2) Preparation of compound 2
[0099] Add 17.8g of anthracene, 5.4g of benzoquinone, and 75mL of mesitylene into a 200mL flask in sequence, reflux and stir for 24 hours, cool to room temperature, and filter. The obtained solid was added into 100 mL of hot xylene, stirred for a while and filtered while hot, and t...
Embodiment 3
[0115] Preparation of target fluorescent compound (p-iodoaniline is used in this example, alkyl chain n=5)
[0116] 1) Synthesis of compound 1a
[0117] Add 1.00g of perylenetetracarboxylic anhydride, 11.2g of p-iodoaniline, and 17g of imidazole into the flask in turn, and under an argon atmosphere, heat to 140°C under strong reflux and stir for 33 hours, then cool the reaction solution to room temperature naturally, add dichloromethane, and then The resulting suspension was filtered with suction, rinsed with methanol and chloroform in order to remove excess p-iodoaniline, and the obtained material was vacuum-dried to obtain brown-red compound 1a;
[0118] Its reaction equation is as follows:
[0119]
[0120] 2) Preparation of compound 2
[0121] Add 17.8g of anthracene, 5.4g of benzoquinone, and 75mL of mesitylene into a 200mL flask in sequence, reflux and stir for 24 hours, cool to room temperature, and filter. The obtained solid was added into 100 mL of hot xylene, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com