Photosensitive chromatographic stationary phase based on silicon substrate modified by azobenzene photosensitive compound
A photosensitive compound and chromatographic stationary phase technology, applied in the field of photosensitive chromatographic stationary phase, can solve the problems of non-in-situ regulation and single property of chromatographic stationary phase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 The method for synthesizing silylated photosensitive compound with 4-hydroxyazophenol as raw material
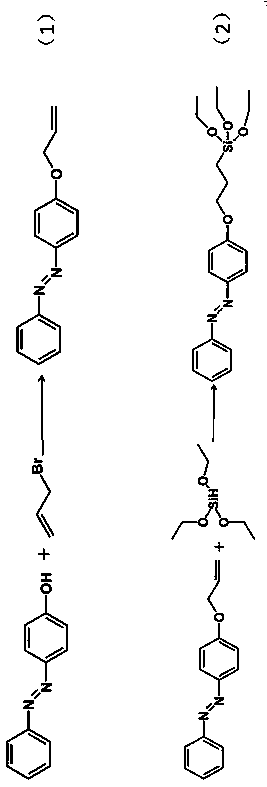
[0036] Dissolve 0.99 g (0.005 mol) of 4-hydroxyazophenol in 12.5 mL of N,N-dimethylformamide, 0.90 g (0.0225 mol) of sodium hydride in 15 mL of N,N-dimethylformamide, and The above two solutions were mixed in a 100 mL round bottom flask, stirred at room temperature for 2 h; 1.82 g (0.015 mol) allyl bromide was added to the above mixed solution, and the reaction temperature was 60 o C, stirred for 24 h; after the reaction was completed, it was extracted with ethyl acetate, washed repeatedly with cold water, and dried at room temperature to obtain an orange-red crude solid product; the crude solid product was dissolved in a small amount of ethanol and crystallized at low temperature to obtain an orange-red fixed product 4 - Allyloxyazobenzene ( figure 1 , response(1));
[0037] 4-allyloxyazobenzene (201 mg, 0.001 mol), triethoxysilane (746 mL, 0.005 mol) ...
Embodiment 2
[0038] Example 2 Using 4-dimethylamino-4'-hydroxyazobenzene as a raw material for the synthesis of silylated photosensitive compounds
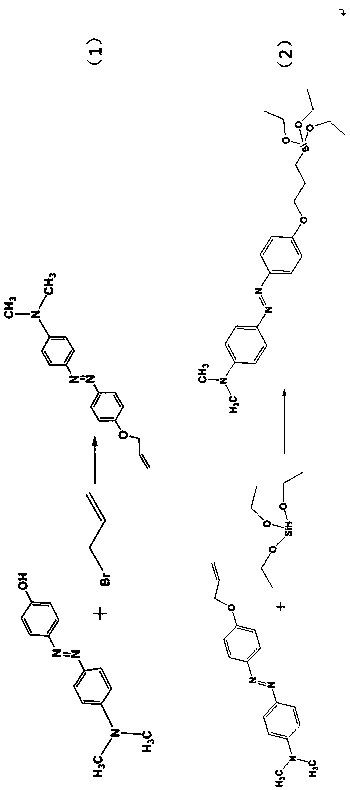
[0039]Dissolve 1.21 g (0.005 mol) of 4-dimethylamino-4'-hydroxyazobenzene in 20 mL of N,N-dimethylformamide, and 0.90 g (0.0225 mol) of sodium hydride in 15 mL of N,N- Dimethylformamide, mix the above two solutions in a 100 mL round bottom flask, stir at room temperature for 2 h; add 1.82 g (0.015 mol) allyl bromide to the above mixture, and react under nitrogen atmosphere temperature 80 o C, stirred for 24 h; after the reaction was completed, it was extracted with ethyl acetate, washed repeatedly with cold water, and dried at room temperature to obtain the crude solid product; the crude solid product was dissolved in a small amount of ethanol and crystallized at low temperature to obtain the intermediate product 4-dimethylamino -4'-allyloxyazobenzene ( figure 2 , response(1));
[0040] 4-Dimethylamino-4'-allyloxyazobenzene (241 mg, 0.001 ...
Embodiment 3
[0041] Example 3 Preparation method of 4-[3-(triethoxysilyl)propoxy]azobenzene photosensitive thin-layer chromatography stationary phase
[0042] Dip silica gel (5 g) into freshly prepared piranha solution (30% H 2 o 2 : 98%H 2 SO 4 =3:7), hydroxylation for 3 h, stirring and dispersing washing with ultrapure water, natural sedimentation, removing the supernatant, repeating the steps of washing and separation until the supernatant is neutral, and vacuum drying at 60°C to obtain hydroxylated silica gel ; Subsequently, 1 g of hydroxylated silica gel was dispersed in 20 mL of toluene, 40 μL of 4-[3-(triethoxysilyl) propoxy]azobenzene was added to the dispersion, and the reaction was refluxed at 80 °C for 16 h. At the end, the product was washed with toluene until the upper layer was colorless, and dried overnight at 80°C in vacuum to obtain a photosensitive thin-layer chromatography stationary phase named 4-[3-(triethoxysilyl)propoxy]azobenzene Photosensitive TLC stationary ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com