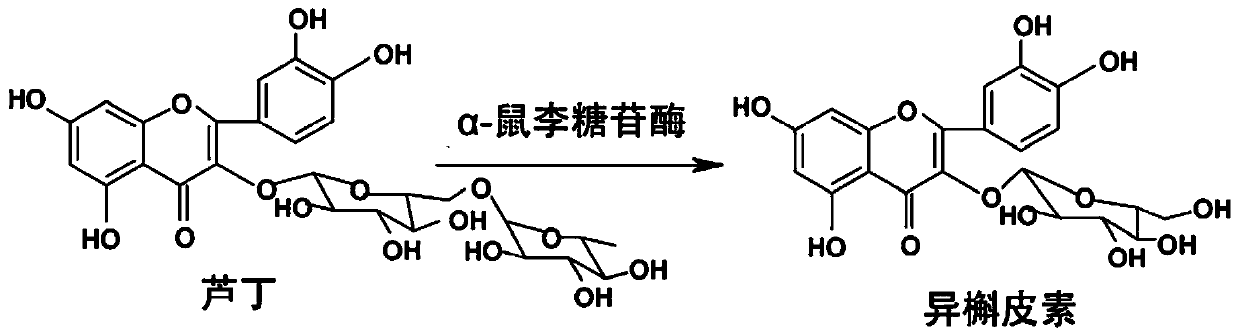
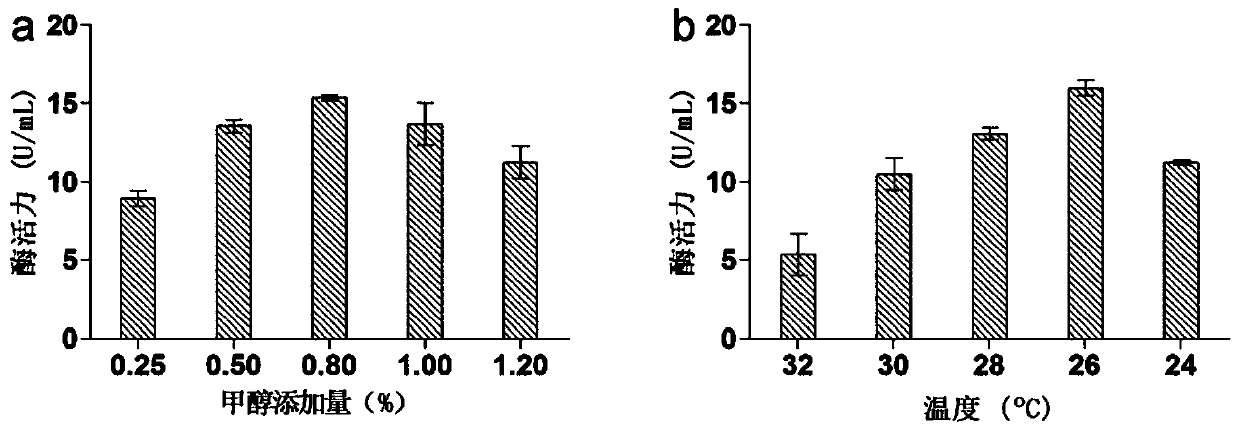
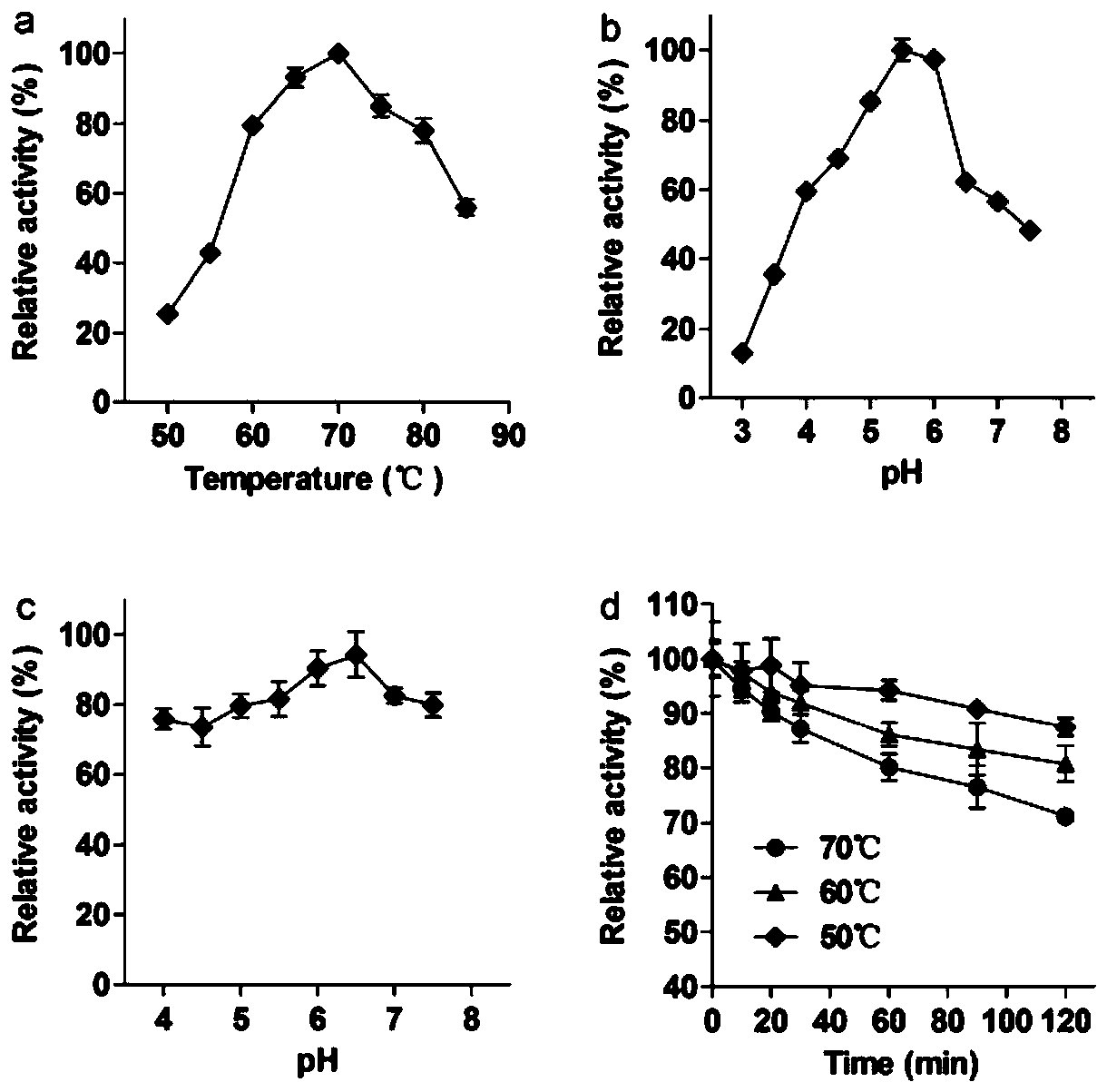
Application of high temperature-resistant alpha-rhamnosidase gene and its transformation of rutin to isoquercetin
A technology of rhamnosidase and rutin, applied in the fields of genetic engineering technology and biomedicine, can solve problems such as single component, and achieve the effects of excellent thermal stability and strong transformation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The induced expression of the preparation method of α-rhamnosidase in the present invention is specifically to cultivate the recombinant bacteria containing MTHRHA gene in BMMY, and collect the supernatant at the end of the enzyme production stage to obtain the required target enzyme protein.
[0029] The present invention also provides a method for enzymatically converting rutin to prepare isoquercetin, specifically, the α-rhamnosidase of the present invention can enzymatically decompose rutin at pH 5.5 and 70°C to prepare isoquercetin white.
Embodiment 1
[0031] Example 1: Construction of recombinant plasmid pPICZαA-MTHRHA
[0032] The primers were designed according to the codon-optimized M.thermophila ATCC 42464 high temperature resistant α-rhamnosidase gene, and the primers were synthesized by Shanghai Bioengineering Co., Ltd. The primer sequences are as follows:
[0033] P1: CCG GAATTC GCTGTTACTCGAACACACCTACA, the underline indicates the EcoR I site (SEQ ID NO.3).
[0034] P2: TGC TCTAGA TTTCCTTAACTGCATAATGGTGA, the Xba I site is underlined, and the stop codon is removed (SEQ ID NO. 4).
[0035] Using the cDNA of M.thermophila ATCC 42464 as a template, carry out PCR amplification, the amplification condition is 95°C, 5min; pause the timer, add Pyrobest polymerase, add 40μL paraffin oil to seal; 30 cycles (94°C, 30s; 58 ℃, 30s; 72℃, 2min); 72℃, 10min; the reaction was stopped, and kept at 4℃. PCR amplification products were purified by gel recovery kit. The DNA molecule of α-rhamnosidase MTHRHA was obtained.
[0036...
Embodiment 2
[0037] Embodiment 2: the preparation of α-rhamnosidase of the present invention
[0038] After linearizing the ScaI restriction endonuclease, the recombinant plasmid pPICZαA-MTHRHA was transformed into Pichia pastoris GS115 host bacteria (purchased from Novagen), and the YPDS plate (LB medium: peptone 20g / L) containing the antibiotic Zeocin (100 μg / mL) , yeast extract 10g / L, glucose 20g / L, sorbitol 1M, agar 15g / L) after culturing at 28°C, pick the ingots into 200mL BMMY medium at 28°C, shake at 200rpm until OD 600 When the concentration is 0.8, add the inducer every 24 hours to a final concentration of 0.5%. After culturing at 30°C for 15 days, use a high-speed refrigerated centrifuge to centrifuge the culture solution at 13,000 rpm for 15 minutes at 4°C, and collect the supernatant as the target enzyme protein .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com