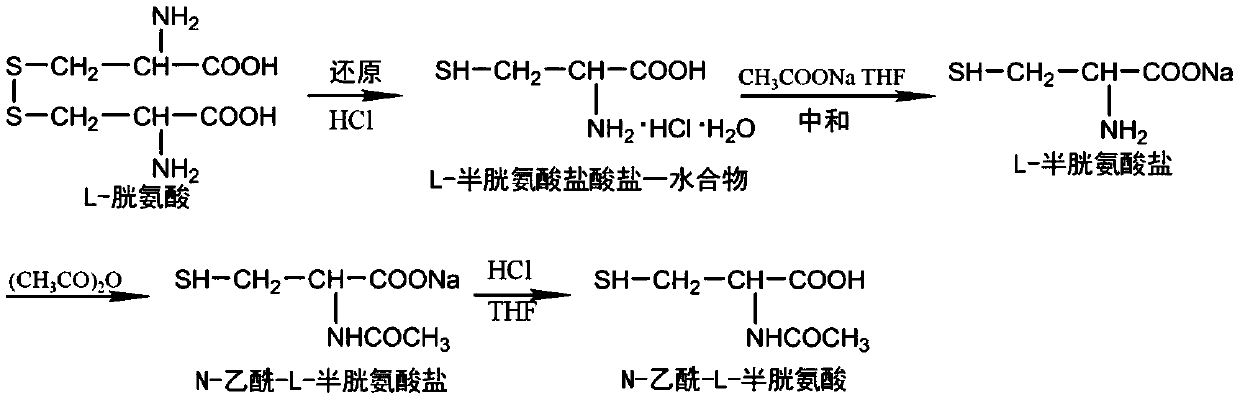
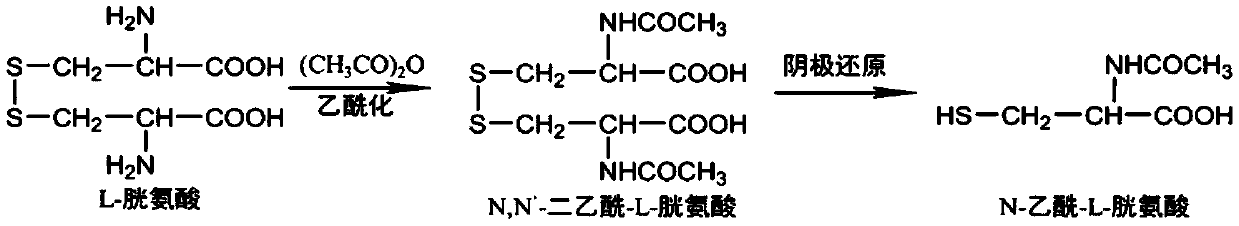
Method for electrochemical synthesis of N-acetyl-L-cysteine from L-cystine
A cysteine and cystine technology, applied in the field of organic electrochemical synthesis, can solve the problems of easy racemization of L-type amino acids, high L-cysteine content, increased cost and investment, etc., and achieve easy production and operation. , The effect of simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] (1) Acetylation of L-cystine to prepare N,N'-diacetyl-L-cystine
[0032] Take 24g L-cystine and dissolve it in 250mL of 1mol L -1 In the sodium hydroxide solution, stir vigorously in an ice water (0°C) bath, then add 40mL of acetic anhydride dropwise, during the process of adding acetic anhydride, continuously add 1mol L -1 Sodium hydroxide, to keep the pH of the reaction solution at about 10, continue stirring for 30min after the addition of acetic anhydride, and then add 1.5mol L -1 hydrochloric acid to adjust the pH to 2. Put the reaction solution in an electrodialyzer to remove salt, when the conductivity of the dilute chamber reaches 500μS cm -1 The electrodialysis was stopped at the following time. Heat the desalinated solution to 60°C, stir and add activated carbon for decolorization, keep warm for 30 minutes for decolorization, and obtain a clear solution after filtration. The solution after electrodialysis was distilled under reduced pressure until all the ...
example 2
[0036] (1) Acetylation of L-cystine to prepare N,N'-diacetyl-L-cystine
[0037] Dissolve 24g of L-cystine into 125mL of 2mol L -1 In the sodium hydroxide solution, stir vigorously in an ice water (0°C) bath, then add 40mL of acetic anhydride dropwise, and continuously add 2mol L of acetic anhydride during the dropwise addition of acetic anhydride-1 Sodium hydroxide, to keep the pH of the reaction solution at about 10, continue stirring for 30min after the addition of the acid anhydride, and then add 3mol L -1 hydrochloric acid to adjust the pH to 2. Put the reaction solution in an electrodialyzer to remove salt, when the conductivity of the dilute chamber reaches 500μS cm -1 The electrodialysis is stopped at the following time. Heat the desalinated solution to 60°C, stir and add activated carbon for decolorization, keep warm for 30 minutes for decolorization, and obtain a clear solution after filtration. The electrodialysis solution was distilled under reduced pressure unti...
example 3
[0041] (1) Acetylation of L-cystine to prepare N,N'-diacetyl-L-cystine
[0042] Take 1200g L-cystine and dissolve it into 6.25L of 2mol L -1 Sodium hydroxide solution, then placed in a double-layer reactor, vigorously stirred in a circulating ice water (0°C) bath, then slowly added 1.5L of acetic anhydride, in the process of dropping acetic anhydride, continuously added 2mol L -1 Sodium hydroxide, to keep the pH of the reaction solution at about 10, continue stirring for 30min after the addition of the acid anhydride, and then add 3mol L -1 hydrochloric acid to adjust the pH to 2. Put the reaction solution in an electrodialyzer to remove salt, when the conductivity of the dilute chamber reaches 500μS cm -1 The electrodialysis is stopped at the following time. Heat the desalinated solution to 60°C, stir and add activated carbon for decolorization, keep warm for 30 minutes for decolorization, and obtain a clear solution after filtration. The electrodialysis solution was dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com