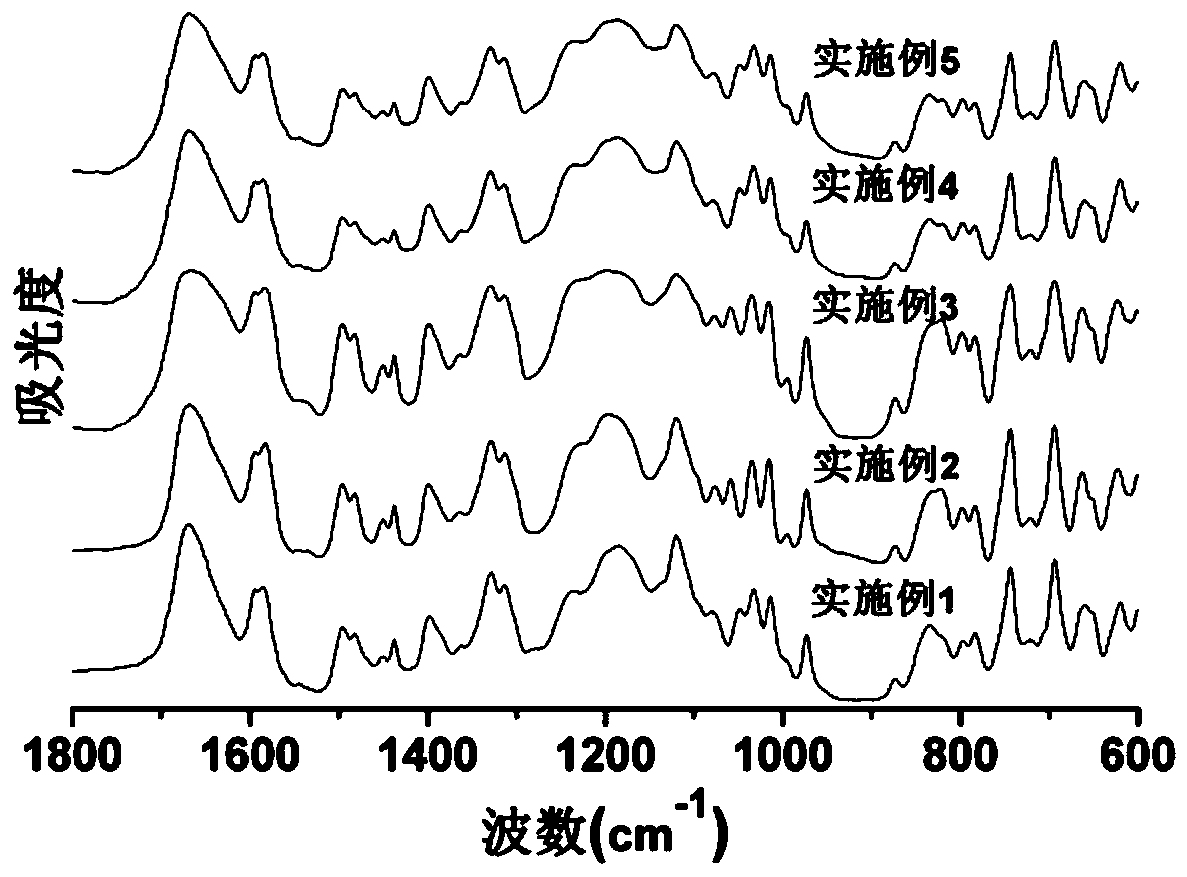
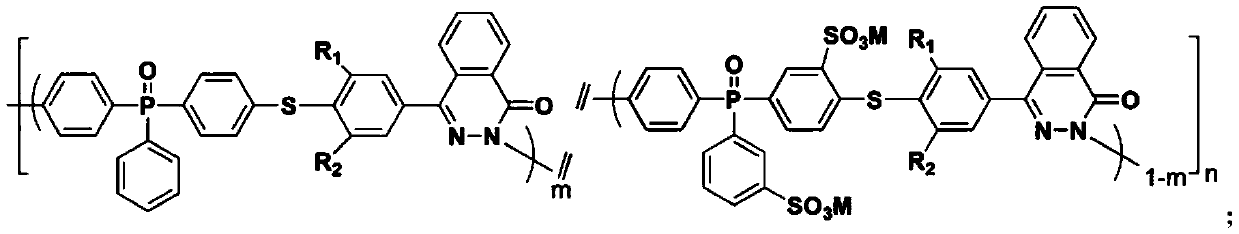
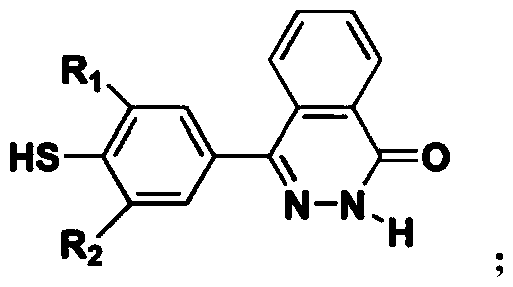
Phthalazinone-containing disulfonated poly(arylene thioether phosphine oxide), and preparation method and application thereof
A technology for disulfonated polyarylene sulfide and naphthalenone, which is applied in the field of disulfonated polyarylene sulfide phosphine oxide and its preparation, can solve problems such as dimensional stability and oxidation resistance that need to be improved, and achieve improved Oxidative stability, low swelling, good high temperature oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] with N 2 Inlet air duct, spherical condenser, water separator, stirring rod, N 2 Add 0.7629g (3.0mmol) 4-(4′-mercapto)phenyl-2,3-naphthalazinone, 0.6128g (1.95mmol) bis(4-halogen Phenyl)phenylphosphine oxide, 0.5443g (1.05mmol) 3-sodium sulfonate-4-halophenyl-3'-sulfonate phenyl-4"-halophenylphosphine oxide, and 0.4561g (3.3mmol ) Anhydrous potassium carbonate. After passing nitrogen for about 15 minutes, add 8 mL of DMAc reaction solvent and 8 mL of toluene (except toluene in the water separator), and the water in the reaction system is brought out by heating and refluxing with toluene at 150 ° C, and drained after 4 hours For the toluene in the reaction system, the temperature was raised to 160°C to continue the reaction until the viscosity of the reaction polymer increased to a certain extent. After the temperature was lowered to 120°C, the reactant was poured into 300mL deionized water, and stirred while pouring to obtain a shallow Yellow filamentous polymer, soak...
Embodiment 2
[0061] with N 2 Inlet air duct, spherical condenser, water separator, stirring rod, N 2 Add 0.7629g (3.0mmol) 4-(4′-mercapto)phenyl-2,3-naphthalazinone, 0.5657g (1.8mmol) bis(4-halogen Phenyl)phenylphosphine oxide, 0.6220g (1.2mmol) 3-sodium sulfonate-4-halophenyl-3'-sulfonate phenyl-4"-halophenylphosphine oxide, and 0.4561g (3.3mmol ) anhydrous potassium carbonate. After passing nitrogen for about 15 minutes, add 8 mL of DMAc reaction solvent and 8 mL of toluene (except for toluene in the water trap), and heat the water in the reaction system to reflux at 150 ° C and take it out through toluene, and drain it after 4 hours Toluene, the temperature is increased to 160°C to continue the reaction until the viscosity of the reaction polymer increases to a certain extent. After the temperature is lowered to 120°C, the reactant is poured into 300mL deionized water, and stirred while pouring to obtain a light yellow filament Polymer, soaked in hot water to remove inorganic salts th...
Embodiment 3
[0064] with N 2 Inlet air duct, spherical condenser, water separator, stirring rod, N 2 Add 0.7629g (3.0mmol) 4-(4′-mercapto)phenyl-2,3-naphthyridine, 0.5185g (1.65mmol) bis(4-halogen Phenyl)phenylphosphine oxide, 0.6998g (1.35mmol) 3-sodium sulfonate-4-halophenyl-3'-sulfonate phenyl-4"-halophenylphosphine oxide, and 0.4561g (3.3mmol ) anhydrous potassium carbonate. After passing nitrogen for about 15 minutes, add 8 mL of DMAc reaction solvent and 8 mL of toluene (except for toluene in the water trap), and heat the water in the reaction system to reflux at 150 ° C and take it out through toluene, and drain it after 4 hours Toluene, the temperature is increased to 160°C to continue the reaction until the viscosity of the reaction polymer increases to a certain extent. After the temperature is lowered to 120°C, the reactant is poured into 300mL deionized water, and stirred while pouring to obtain a light yellow filament Polymer, soaked in hot water to remove inorganic salts th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com