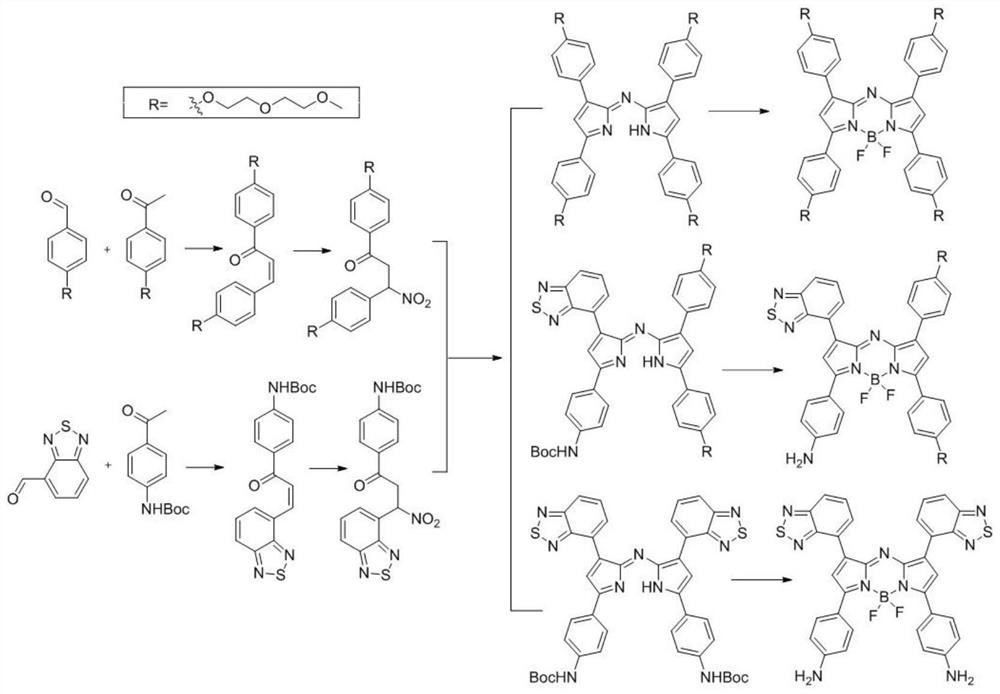
Aza-bodipy derivatives with photothermal effect and their synthesis and application
A technology of photothermal effect and derivatives, which is applied in the field of biochemistry, can solve the problems of high price, and achieve the effect of low cost, obvious effect and high photothermal conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of compounds of formula Ⅰ-1
[0038]
[0039] Synthesis of Compound A
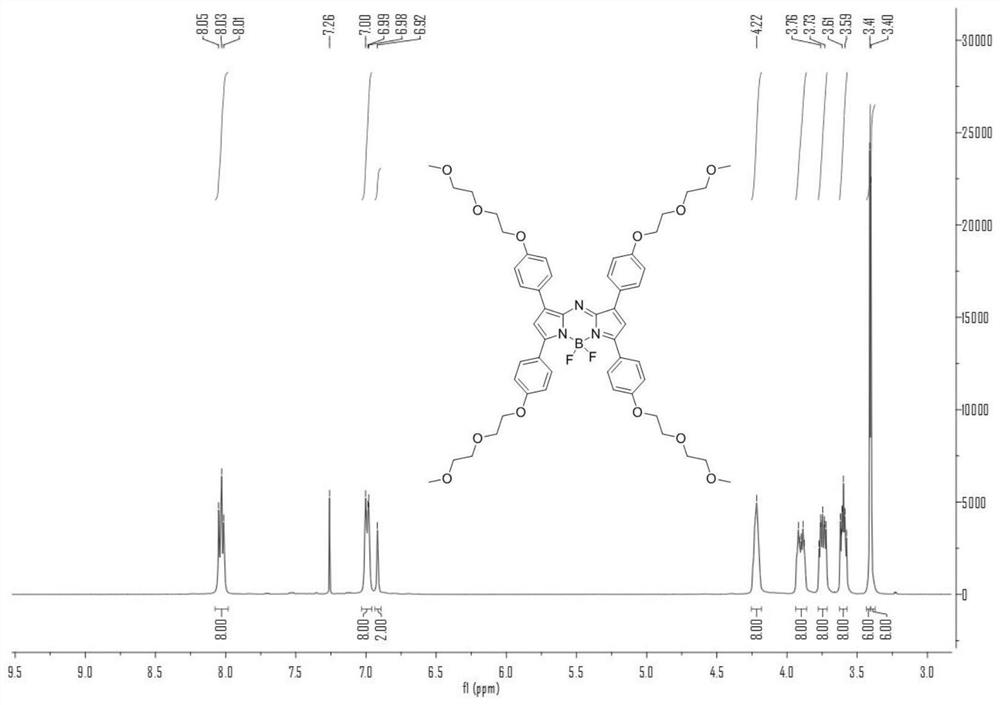
[0040] N 2 Under gas protection, weigh compound 1 (1.0g, 4.46mmol) and compound 2 (1.5g, 6.23mmol), add it to a 200mL round bottom flask, dissolve it with an appropriate amount of ethanol, add 15mL of 10% sodium hydroxide solution, and Stir overnight. Half-time observation, until the reaction is complete, dichloromethane extraction, combined organic phase, anhydrous Na 2 SO 4 Dry, evaporate the organic phase to dryness, and separate by column chromatography. The ratio of developer is DCM:EA=20:1. The product A is a yellow oily liquid with a yield of 72%. 1 H NMR (400MHz, CDCl 3 )δ=8.02(d, J=8Hz, 2H), 7.77(d, J=16Hz, 1H), 7.59(d, J=8Hz, 2H), 7.43(d, J=16Hz, 1H), 6.97(dd ,J=8Hz,4H),4.23(t,J=4Hz,2H),4.19(t,J=4Hz,2H),3.89(q,J=4Hz,4H),3.75-3.72(m,4H), 3.60-3.58(m,4H),3.40(s,6H).
[0041] Synthesis of Compound B
[0042] N 2 Under gas protection, weigh compound A (1.0g, 2.25mmol) an...
Embodiment 2
[0048] Synthesis of compounds of formula Ⅰ-2
[0049]
[0050] Synthesis of Compound D
[0051] N 2 Under gas protection, compound 1 (1.0 g, 4.46 mmol) and compound 3 (2.5 g, 10.63 mmol) were weighed, dissolved in an appropriate amount of ethanol, 15 mL of 10% sodium hydroxide was added, and stirred overnight at room temperature. After the reaction was completed, DCM was extracted, the organic phases were combined, dried over anhydrous Na2SO4, evaporated to dryness, and separated by column chromatography. The developer ratio was PE:EA=8:1, and the product was a bright yellow colloidal solid with a yield of 76.2 %. 1 H NMR (400MHz, CDCl3) δ=8.01(d, J=8Hz, 2H), 7.78(d, J=8Hz, 1H), 7.59(d, J=8Hz, 2H), 7.49(d, J=8Hz, 2H), 7.42(d, J=8Hz, 1H), 4.19(t, J=4Hz, 2H), 3.88(q, J=4Hz, 4H), 3.74-3.72(m, 4H), 3.60-3.58(m ,4H),3.40(s,3H),1.54(s,9H).
[0052] Synthesis of Compound E
[0053] N 2 Under gas protection, compound D (1.55g, 3.51mmol) and nitromethane (2.8mL, 52.3mmol) wer...
Embodiment 3
[0059] All in vivo experiments in the present invention abide by the rules and regulations for the feeding and use of experimental animals. The tumor-bearing mice used in the experiments were purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd., and were raised in a sterile mouse cage in a laminar flow fume hood in a sterile room. Autoclaved steam treated water and food were fed.
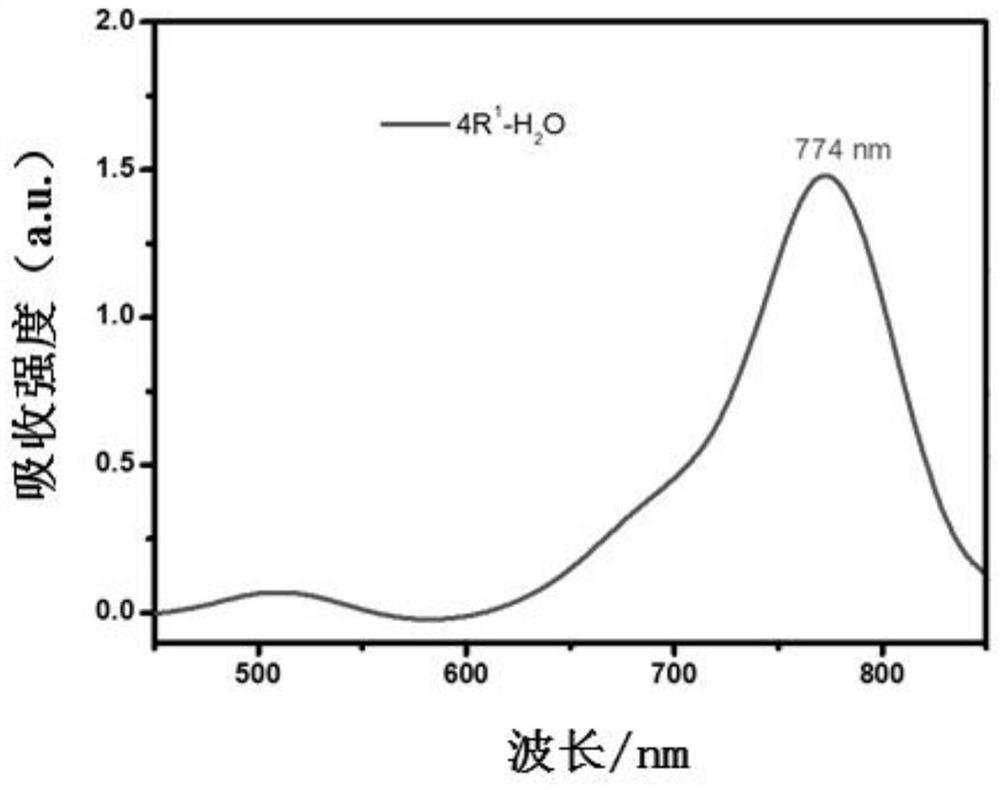
[0060] Inject aza-BODIPY of formula Ⅰ-2 subcutaneously in the tumor site of tumor-bearing mice, with a dose of 100 nmol at a time, and use laser irradiation after injection (remarks: laser wavelength: 785nm, power density: 1.57Wcm -2 ), while using thermal imaging to monitor the temperature in real time, the body temperature of the tumor site can rise above 70°C after 10 minutes of irradiation (such as Figure 6 Shown), the size of the mouse tumor was recorded every day, and it was found that the administered probe was cured after 10 days without recurrence, while the tumor volume of the mou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com