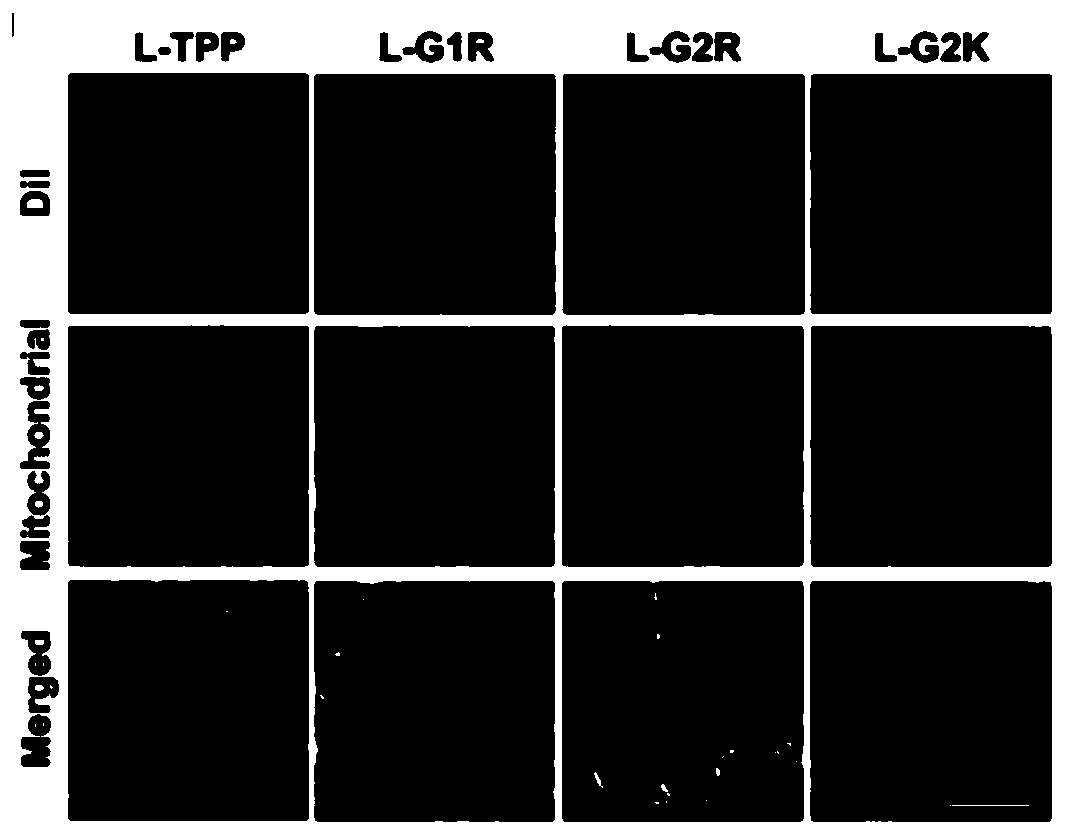
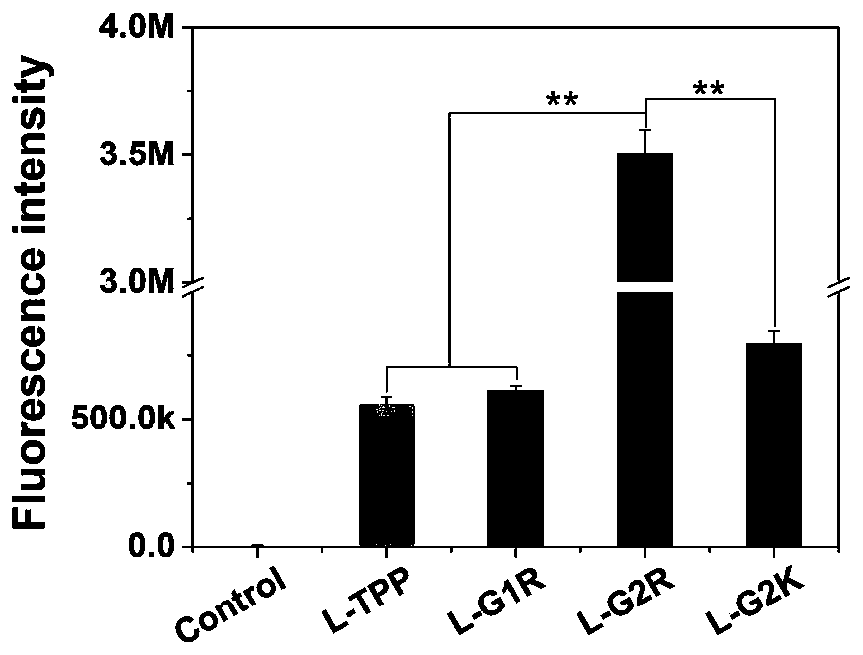
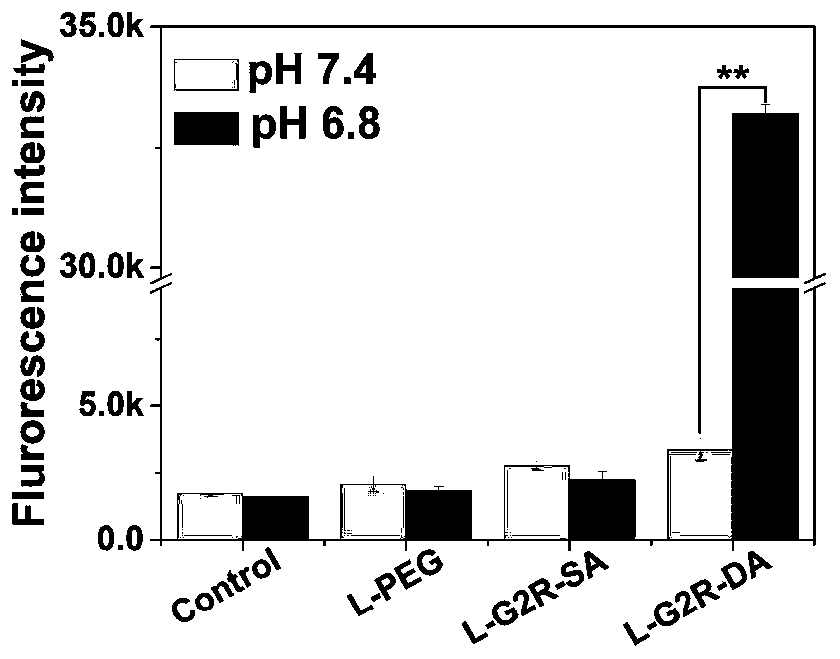
Phospholipid targeting mitochondria and application
A technology of mitochondria and phospholipids, applied in the field of mitochondria-targeted phospholipids, pH-sensitive and mitochondria-targeted phospholipids, can solve the problems of restricting clinical transformation and application, the synthesis process of poorly permeable polypeptides, and the non-specific toxicity of cationic substances. Effect of cellular uptake, reduction of non-specific toxicity, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In this embodiment, the mitochondria-targeted phospholipid is distearoylphosphatidylethanolamine-arginine (G1R), and its structural formula is:
[0052]
[0053] Its preparation method is as follows:
[0054] Take 0.75g (0.75mmol) of DSPE and dissolve it in 15mL of anhydrous chloroform under the protection of nitrogen. Another 1 mL of DIPEA (6.00 mmol) was stirred into the DSPE solution at 0°C. Then 0.79g Boc-Arg(Pbf)-OH (0.79mmol), 0.2g HOBT (0.2mmol) and 0.57g HBTU (1.5mmol) were co-dissolved in 2.5mL of DMF, added to the reaction flask containing DSPE, and then in The reaction was continued for 24 hours at room temperature. After the reaction was finished, the reaction product was repeatedly washed several times with saturated sodium bicarbonate, sodium bisulfate and sodium chloride solution, and then dried with magnesium sulfate for 2 hours. Finally, after the solvent was removed, it was purified by silica gel column chromatography, and the eluent was dichlorome...
Embodiment 2
[0056] In this embodiment, the mitochondria-targeted phospholipid is distearoylphosphatidylethanolamine-second-generation arginine (G2R), and its structural formula is:
[0057]
[0058] Its preparation method is as follows:
[0059] Weigh H-Lys-OMe·2HCl 2.00g (8.6mmol), Boc-Arg(Pbf)-OH 13.56g (13.56mmol), HBTU 9.78g (25.8mmol), HOBT 3.36g (3.36mmol), under nitrogen protection were dissolved together in 50 mL of anhydrous DMF, and then 11.4 mL of DIPEA (68.8 mmol) was added to the above mixture in an ice-water bath, and the reaction was stirred at room temperature for 48 h. Then, the products were washed with saturated NaHCO 3 , NaHSO 4 and NaCl solution several times. The product was then mixed with MgSO 4 Mix for 2h to remove water. After removing the solvent, the product was purified by silica gel column chromatography, the eluent was dichloromethane:methanol (12:1, v / v), and the product was collected. Weigh 8.00 g (6.8 mmol) of the product in 100 mL of methanol so...
Embodiment 3
[0061] In this embodiment, the mitochondria-targeted phospholipid is distearoylphosphatidylethanolamine-second-generation lysine (G2K), and its structural formula is:
[0062]
[0063] Its preparation method is as follows:
[0064] H-Lys-OMe.2HCl (1.00g, 4.3mmol), Boc-L-lys(Boc)-OH (4.46g, 12.9mmol), HBTU (4.89g, 12.9mmol), HOBT (1.68g, 12.9mmol ) was dissolved in anhydrous DMF (25 mL). Add DIPEA (5.7mL, 34.4mmol) in ice-water bath and stir at room temperature for 48 hours, then wash with saturated NaHCO 3 , NaHSO 4 , NaCl solution to wash the mixed solution several times. with MgSO 4 After drying for 2 h and removing the solvent, the product was purified by silica gel column chromatography (DCM / MeOH, 12 / 1, v / v). Take it (4.00g, 3.4mmol) and place it in 50mL NaOH in MeOH (1mol / L) solution for 4h to expose the carboxyl group. After removal of MeOH, the mixture was dissolved in H 2 O, adjust to neutral pH. Extracted with DCM, MgSO 4 Dry for 2h. The product (1.81g, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com