Fecal occult blood detection kit
A detection kit and fecal occult blood technology, applied in the field of medical detection, can solve problems such as high detection cost, poor detection repeatability, and reduced sensitivity, and achieve the effect of simple and easy-to-obtain raw materials, reduce detection cost, and ensure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Verifying the influence of reagent 1 and reagent 2 on the stability of fecal occult blood detection
[0040] Reagent 1 and reagent 2 were respectively prepared using the formulas of experimental groups 1-3 and the control group, and the solvent was water, and the antibodies of each group were labeled on the latex microspheres according to the conventional chemical coupling method.
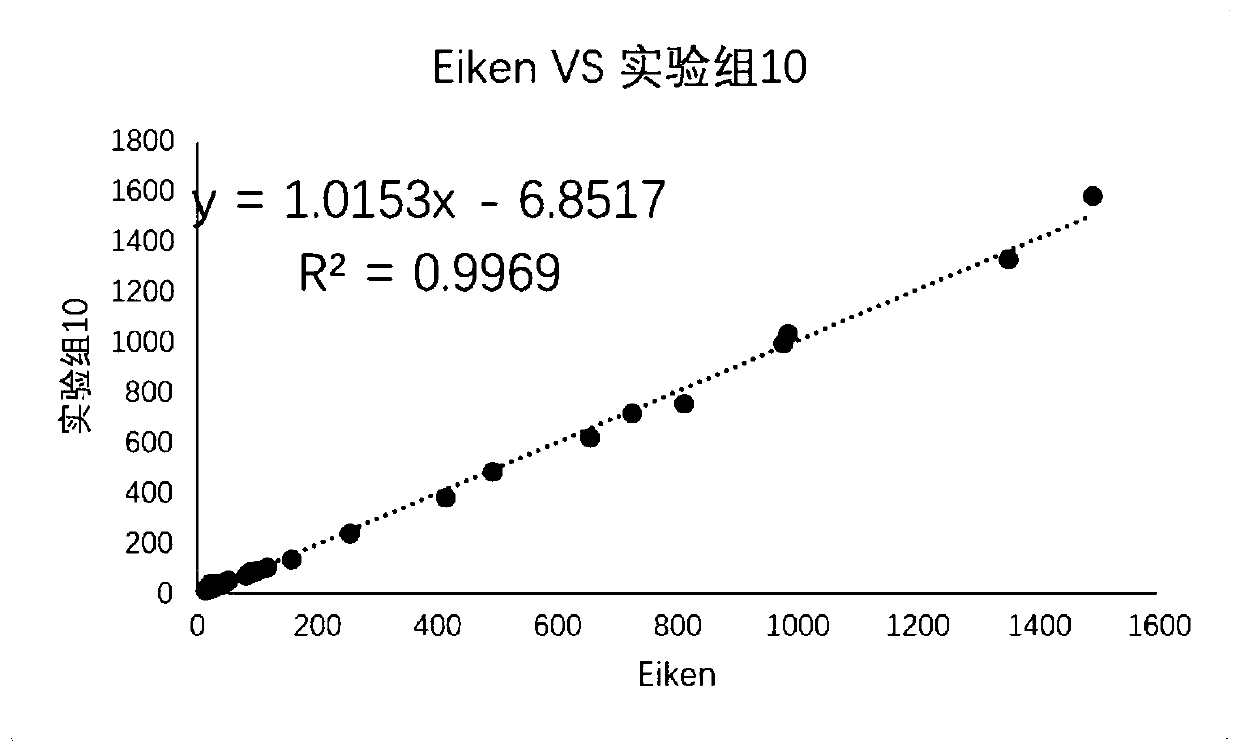
[0041] Experimental group 1
[0042] Reagent 1: 4-hydroxyethylpiperazineethanesulfonic acid 23.8g / L, sodium chloride 70.1g / L, tris(2-carboxyethyl)phosphine (TCEP) 1g / L, glutathione 1g / L, Glutamic acid 1g / L, arginine 0.1g / L, cysteine 0.1g / L, disodium edetate 1g / L, bovine serum albumin (IgG free grade) 1g / L, polyethylene Diol 6000 10g / L, Tween-20 2ml / L, blocking agent HBR-X (Scantibody Company) 2ml / L, sodium hydroxide to adjust PH6.90, preservative ProClin300 0.6ml / L.
[0043]Reagent 2: 12.2 g / L of trismethylaminopropanesulfonic acid, 45 g / L of trehalose, 1.8 g / L of 300 nm latex ...
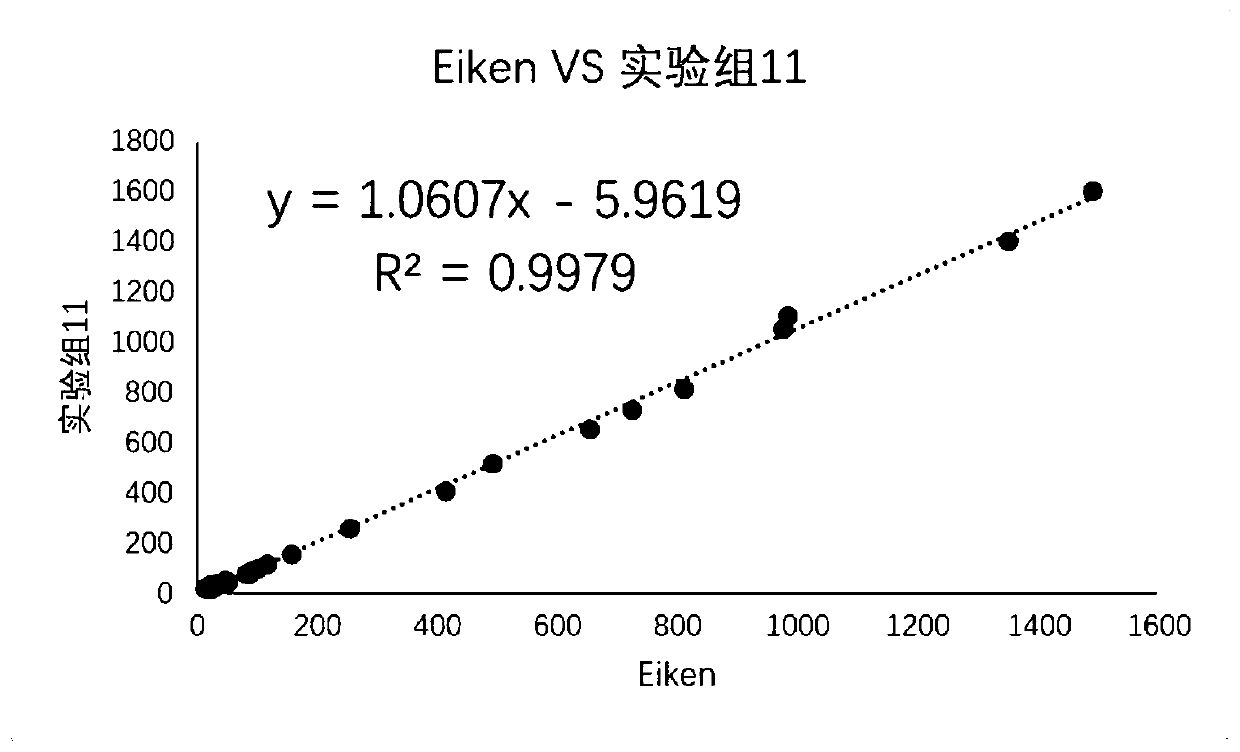
Embodiment 2
[0061] Embodiment 2 verifies the impact of sample diluent on sample storage stability
[0062] Experimental group 4 sample diluent: 4-hydroxyethylpiperazineethanesulfonic acid 11.9 / L, sodium chloride 46.8g / L, trehalose 30g / L, sucrose 60g / L, sodium lauryl polyoxyethylene ether sulfate 10g / L L, tris(2-carboxyethyl)phosphine (TCEP) 1g / L, glutathione 1g / L, glutamic acid 1g / L, arginine 0.1g / L, cysteine 0.1g / L, Disodium edetate 1g / L, bovine serum albumin (IgGfree grade) 1g / L, sodium hydroxide to adjust PH6.90, preservative ProClin300 0.6ml / L; solvent is water.
[0063] Experimental group 5 sample diluent: 4-hydroxyethylpiperazineethanesulfonic acid 23.8g / L, sodium chloride 58.4g / L, trehalose 30g / L, sucrose 80g / L, sodium lauryl polyoxyethylene ether sulfate 10g / L, tris(2-carboxyethyl)phosphine (TCEP) 1g / L, glutathione 2g / L, glutamic acid 2g / L, arginine 0.2g / L, cysteine 0.2g / L , disodium edetate 2g / L, bovine serum albumin (IgG free grade) 2g / L, sodium hydroxide adjusted to 7.20...
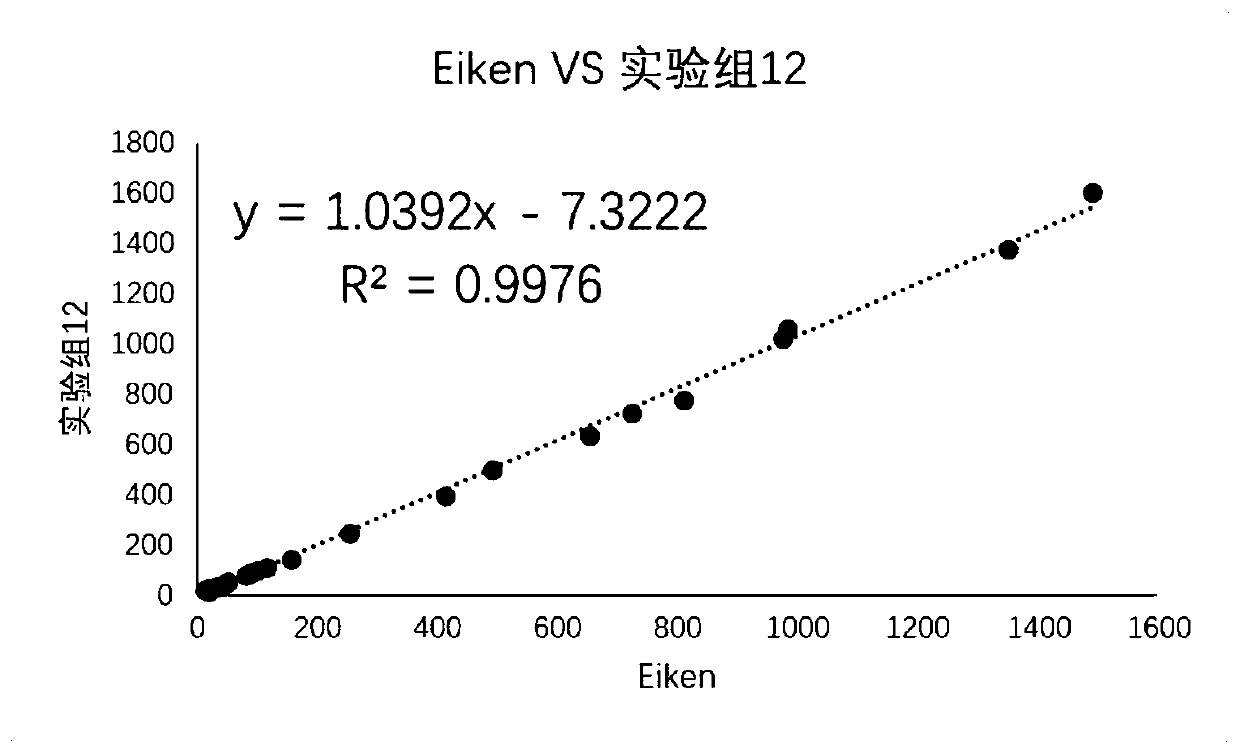
Embodiment 3
[0073] The impact of embodiment 3 calibrator / quality control product background buffer solution on storage stability
[0074] Experimental group 7 calibrator / quality control background buffer: 4-hydroxyethylpiperazineethanesulfonic acid 5.96g / L, sodium chloride 2.9g / L, trehalose 45g / L, maltose 10g / L, three (2 -Carboxyethyl)phosphine (TCEP) 1g / L, glutathione 1g / L, glutamic acid 1g / L, arginine 0.1g / L, cysteine 0.1g / L, ethylenediaminetetraacetic acid Disodium 1g / L, mannitol 2g / L, fetal bovine serum (mycoplasma-free Chlamydia grade) 50ml / L, sodium hydroxide to adjust PH6.90, preservative ProClin300 0.6ml / L, solvent is water.
[0075] Experimental group 8 calibrator / quality control background buffer: 4-hydroxyethylpiperazineethanesulfonic acid 11.9g / L, sodium chloride 5.8g / L, trehalose 45g / L, maltose 20g / L, three (2 -Carboxyethyl)phosphine (TCEP) 1g / L, glutathione 2g / L, glutamic acid 2g / L, arginine 0.2g / L, cysteine 0.2g / L, ethylenediaminetetraacetic acid Disodium 2g / L, mannit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com