Temperature-sensitive chiral amino acid copper complex catalyst and preparation method thereof
A technology of chiral amino acid and amino acid copper, which is applied in the direction of organic compound/hydride/coordination complex catalyst, preparation of organic compound, physical/chemical process catalyst, etc., can solve the problem of difficult catalyst recovery, environmental pollution, water phase catalysis Low efficiency and other problems, to achieve the effects of large-scale industrial production, safe operation, and improvement of catalytic reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
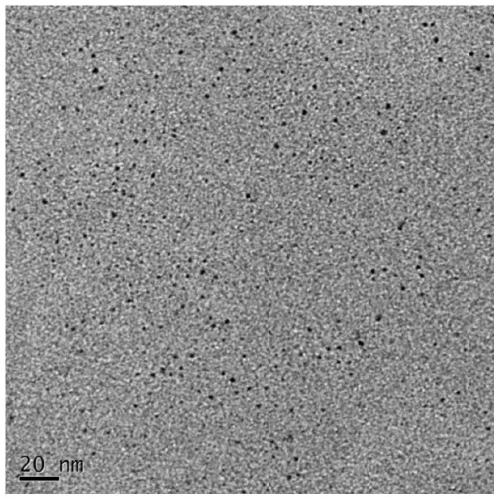
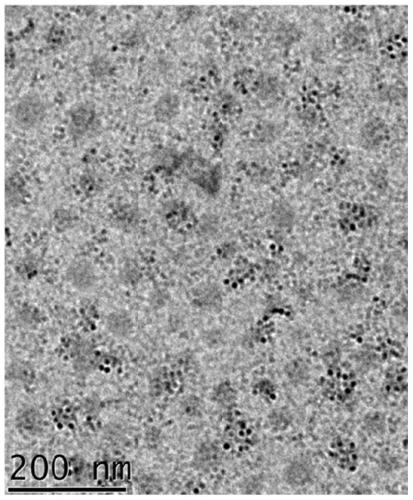
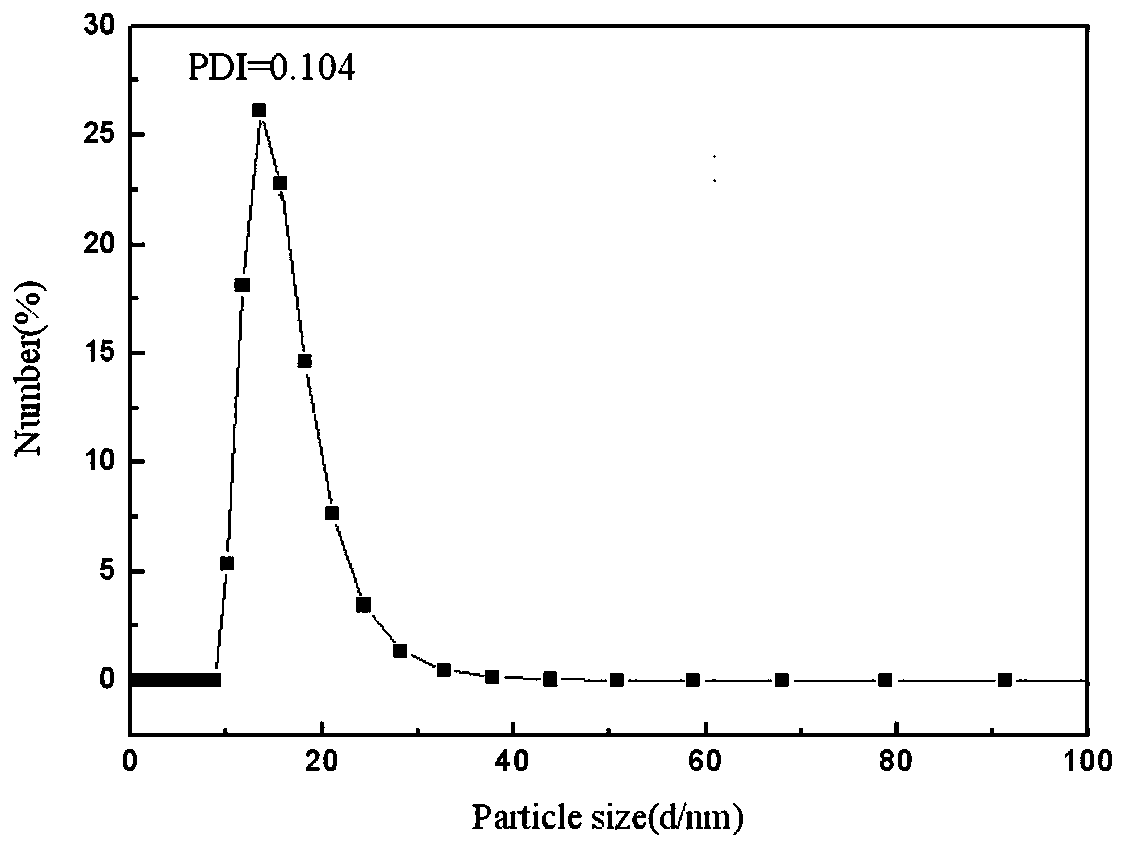
Image
Examples
Embodiment 1
[0049] Cu II -PN 50 A 5 The preparation method comprises steps:
[0050] (1) Synthesis of chiral amino acid compounds containing carbon-carbon double bonds
[0051] Reaction 1
[0052]
[0053] In the above reaction formula 1, R 3 is a hydrogen atom, R 4 is benzyl.
[0054] The specific operation process is to add 10mmol of L-phenylalanine to a 100mL dry three-necked flask, and add 20mL of anhydrous dichloromethane and 11mmol of triethylamine to it, and place the reaction flask in an ice-water bath for magnetic stirring. After the liquid was cooled to 0° C., 30 mL of acryloyl chloride (11 mmol) in dichloromethane solution was slowly added dropwise therein within 30 min. After the dropwise addition, when no steam emerges from the solution, slowly raise the reaction temperature to room temperature, and continue the reaction for 2 to 8 hours. After the reaction was completed, 10 mL of saturated NH 4 Cl solution, CH 2 Cl 2 (2×5ml) to extract the aqueous layer, and th...
Embodiment 2
[0062] The Cu that embodiment 1 obtains II -PN 50 A 5 Used to catalyze the Henry addition reaction; that is, in aqueous medium, aldehydes and nitroalkanes are in Cu II -PN 50 A 5 Under the catalysis of the catalyst, the asymmetric Henry addition reaction is carried out to obtain the β-nitro alcohol compound.
[0063] Aldehyde compounds have the structure of general formula (8); nitroalkanes have the structure of general formula (9); β-nitroalcohol compounds have the structure of general formula (10).
[0064] R 5 -CHO formula (8)
[0065] R 6 -NO 2 Formula (9)
[0066]
[0067] Reaction 3
[0068]
[0069] In this embodiment, the aldehyde compound is benzaldehyde, and the nitroalkane is nitromethane. The specific catalytic process is to add 0.5mmol% catalyst Cu in a 10mL reaction flask. II -PN 50 A 5 , 1mL H 2 O dissolves the catalyst, then adds 1 mmol of substrate (benzaldehyde) and 3 mmol of nitromethane to it, and stirs the reaction at 25°C. Thin-layer ...
Embodiment 3
[0078] The thermosensitive type chiral amino acid copper complex catalyst Cu that embodiment 1 obtains II -PN 50 A 5 Catalysis on different substrates.
[0079] Catalyst Cu II -PN 50 A 5 And the traditional amino acid copper catalyst is used to catalyze the reaction of other aldehydes and nitroalkanes, and the results are shown in Table 2 below:
[0080] Table 2
[0081]
[0082] As can be seen from the table, after 18 hours of reaction, the catalyst Cu II -PN 50 A 5The catalytic effect of the catalyst is significantly better than that of traditional catalysts, and has huge advantages in yield and ee value. This type of catalyst shows good catalytic effect in Henry's asymmetric addition reaction in water phase, and can solve the problem of difficult mass transfer in water phase reaction in industry.
[0083] The characterization data of some products are as follows:
[0084] (R)-1-phenyl-2-nitroalcohol: colorless oil, separated by silica gel column chromatography...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com