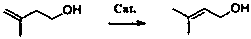Method for synthesizing 3-methyl-2-buten-1-ol from 3-methyl-3-buten-1-ol through trickle-bed transposition
A technology of isopentenol and trickle bed, which is applied in the direction of chemical instruments and methods, isomerization preparation, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problem of high reaction temperature and reaction time of gas phase isomerization Harsh conditions, low selectivity, etc., to achieve the effect of simple catalyst components, mild reaction conditions, and high product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 20g of 10%Ni / Al to a trickle bed reactor with an inner diameter of 12mm and a length of 40cm 2 o 3 Catalyst, the particle size of the catalyst is 10-20 mesh, start filling from the bottom of the reaction tube, the filling height is 25cm, and the filling density is 0.7g / cm 3 . Feed the raw material 3-methyl-3-buten-1-ol and oxygen continuously into the trickle bed reactor equipped with catalyst, at the reaction temperature of 50°C and the space velocity of 1.0 h -1 , the reaction was carried out under the condition that the oxygen-oil ratio was 0.05, and the liquid product was detected and analyzed by gas chromatography, the conversion rate was 70.1%, the selectivity was 98.8%, and the purity of the product prenol was 99.6% after the reaction liquid was rectified. . The catalyst is operated for a long period, and after 1000 hours, the conversion rate is 69.6-70.2%, the selectivity is 98.5-99.0%, and the purity of the product isopentenol is 99.5-99.6%, that is, the...
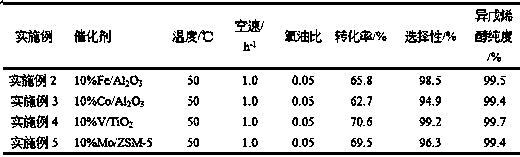
Embodiment 2-5
[0051] On the basis of Example 1, the type of catalyst was changed, and the results are shown in Table 1. Each catalyst was operated for a long period of time. After 1000 hours, the conversion rate fluctuated by ±0.3%, the selectivity fluctuated by ±0.2%, and the purity of the product isopentenol fluctuated by ±0.1%, that is, the catalyst had good stability.
[0052] Table 1 Effect of catalysts on reaction results
[0053]
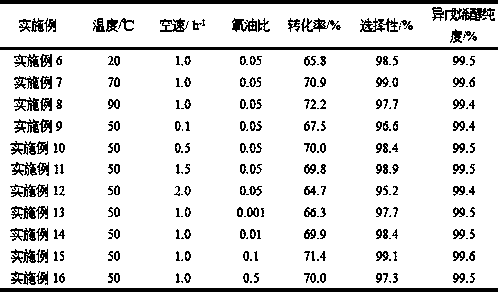
Embodiment 6-16
[0055] On the basis of Example 1, with 10%V / TiO 2 As a catalyst, change the reaction conditions (reaction temperature, space velocity, oxygen-oil ratio), and the results are shown in Table 2. The catalysts in Table 2 were operated for a long period of time under different corresponding reaction conditions. After 1000 hours, the conversion rate fluctuated ±0.3%, the selectivity fluctuated ±0.2%, and the purity of the product prenol fluctuated ±0.1%, that is, the catalyst had Very good stability.
[0056] Table 2 Effect of reaction conditions on reaction results
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com