Method for catalytically dehydrogenating and oxidizing aryl secondary alcohol into ketone
A technology for catalytic dehydrogenation and aryl secondary alcohols, applied in chemical instruments and methods, dehydrogenation preparation, carbon-based compound preparation, etc., can solve problems such as slow disappearance or decomposition, pollution of the environment by useless by-products, instability, etc., to achieve Ease of handling, great application potential, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
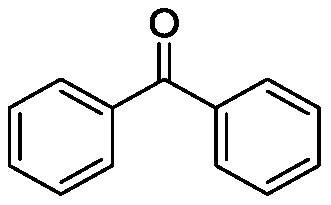
[0026] Taking the preparation of the following formula compound benzophenone as an example, the raw materials used and the preparation method thereof are as follows:
[0027]
[0028] In the reaction tube, add p-toluenesulfonic acid (0.3mmol, 0.50g), tert-butyl chloride (0.6mmol, 0.030mL), benzhydryl alcohol (0.6mmol, 0.11g) in dichloroethane (0.5mL) , stir well at room temperature, then stir and heat up to 60°C, stop the reaction after 2 hours of reaction, add 2mL saturated aqueous sodium bicarbonate solution to the system, extract three times with 10mL ethyl acetate, combine the organic phases, and dry with anhydrous sodium sulfate , filtered, concentrated, and separated by column chromatography (hexane / EtOAc) to obtain a white solid product with an isolated yield of 83%. The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data are as follows: 1 H NMR (600MHz, CDCl 3 )δ7.56(dd, J=8.4,1.2Hz...
Embodiment 2
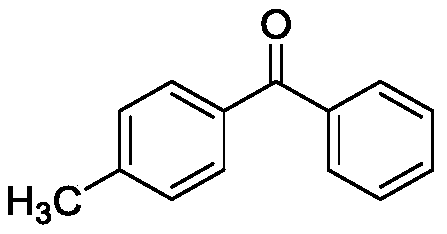
[0030] Taking the preparation of the following formula compound 4-methylbenzophenone as an example, the raw materials used and the preparation method thereof are as follows:
[0031]
Embodiment 3
[0033] Example 3
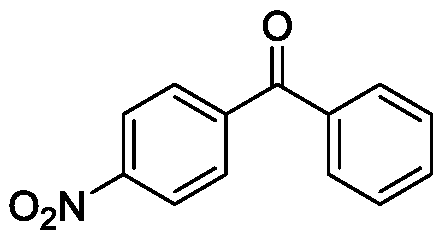
[0034] Taking the preparation of the following formula compound 4-nitrobenzophenone as an example, the raw materials used and the preparation method thereof are as follows:
[0035]
[0036] In embodiment 3, the diphenyl alcohol used is replaced with equimolar 4-nitrobenzophenone, and other steps are identical with embodiment 1, is prepared into white solid 4-nitrobenzophenone, and its isolated yield is 72%, the characterization data is: 1 H NMR (600MHz, CDCl 3 )δ8.26(d, J=8.2Hz, 2H), 7.92(d, J=8.1Hz, 2H), 7.82–7.77(m, 2H), 7.57(d, J=7.3Hz, 1H), 7.47( t,J=7.7Hz,2H); 13 C NMR (151MHz, CDCl 3 )δ194.8, 149.1, 142.9, 136.3, 133.5, 130.7, 130.2, 128.7, 123.6
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com