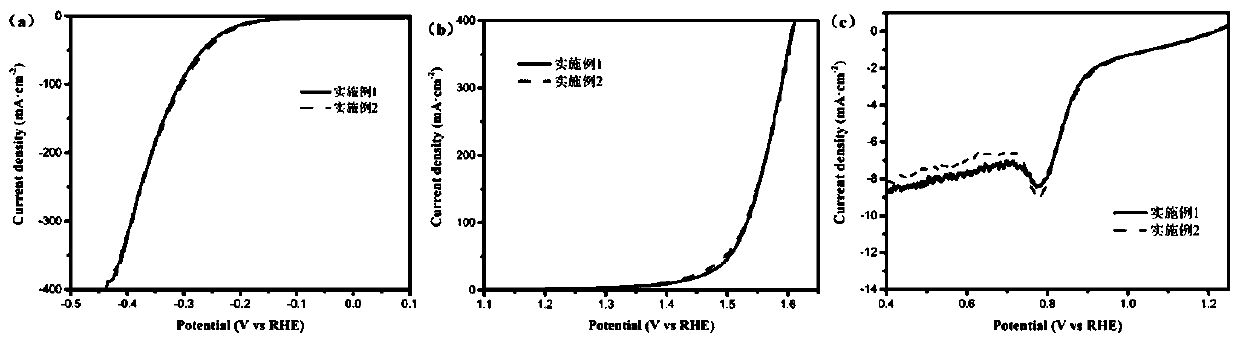
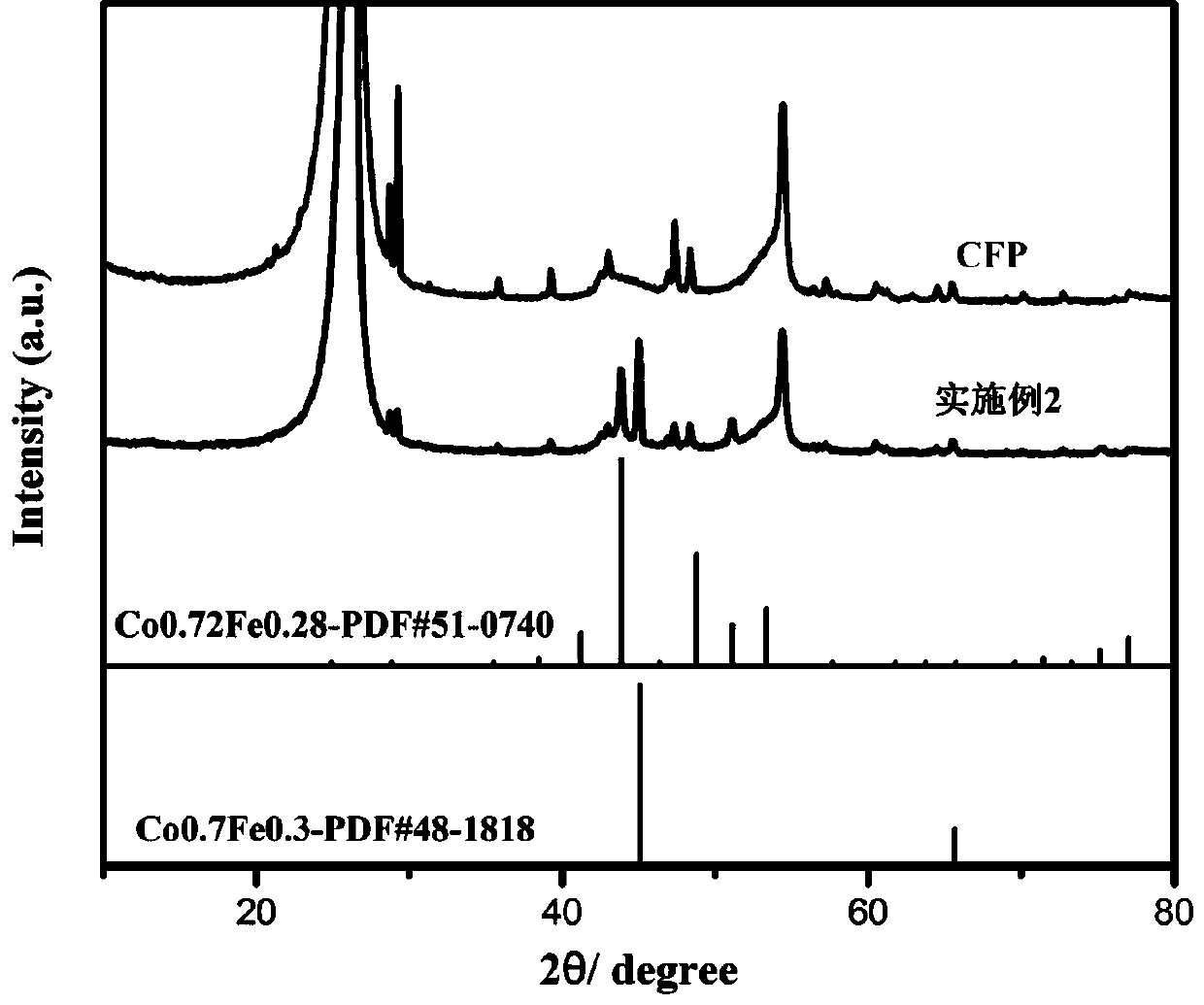
Preparation method of nitrogen-phosphorus co-doped carbon nanotube coated ferrocobalt bimetallic alloy in-situ electrode
A carbon nanotube, in-situ electrode technology, applied in battery electrodes, nanotechnology, nanotechnology and other directions, can solve the problems of inability to meet the requirements of high-energy electronic products, low effective capacity of zinc-air batteries, and low catalytic activity of cathode catalysts, etc. Achieve the effect of enhancing catalytic active sites, improving OER performance, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] At room temperature, dissolve 0.58g of cobalt nitrate and 1.31g of 2-methylimidazole in 40mL of deionized water respectively. After mixing the two solutions, immerse in carbon paper. After standing for 4 hours, take out the carbon paper and rinse it with deionized water and absolute ethanol. Dry it for later use, and grow in situ on the surface of carbon paper to obtain a sheet-like MOF precursor. The above-mentioned carbon paper prepared for growing MOF was immersed in an aqueous solution of ferrous sulfate with a concentration of 0.05 mol / L. After immersion for 8 minutes, the sample was repeatedly rinsed with deionized water and then dried. The carbon paper was further placed in a polytetrafluoroethylene reactor containing 1 g of triphenylphosphine, and the carbon paper was taken out after reacting at 100° C. for 12 hours. Put the carbon paper into one end of the vacuum tube furnace, put 10g of urea into the other end of the vacuum tube furnace, 2 Annealing reaction ...
Embodiment 2
[0026] At room temperature, dissolve 0.58g of cobalt nitrate and 1.31g of 2-methylimidazole in 40mL of deionized water respectively. After mixing the two solutions, immerse in carbon paper. After standing for 4 hours, take out the carbon paper and rinse it with deionized water and absolute ethanol. Dry it for later use, and grow in situ on the surface of carbon paper to obtain a sheet-like MOF precursor. The carbon paper for growing MOF prepared above was immersed in an aqueous solution of ferrous sulfate with a concentration of 0.05 mol / L. After immersion for 15 minutes, the sample was repeatedly rinsed with deionized water and then dried. The carbon paper was further placed in a polytetrafluoroethylene reactor containing 1 g of triphenylphosphine, and the carbon paper was taken out after reacting at 100° C. for 12 hours. Put the carbon paper into one end of the vacuum tube furnace, put 10g of urea into the other end of the vacuum tube furnace, 2 Annealing reaction at 700° C...
Embodiment 3
[0035] At room temperature, dissolve 0.58g of cobalt nitrate and 1.31g of 2-methylimidazole in 40mL of deionized water respectively. After mixing the two solutions, immerse in carbon paper. After standing for 4 hours, take out the carbon paper and rinse it with deionized water and absolute ethanol. Dry it for later use, and grow in situ on the surface of carbon paper to obtain a sheet-like MOF precursor. The above-mentioned carbon paper for growing MOF was immersed in an aqueous solution of ferrous sulfate with a concentration of 0.02 mol / L. After immersion for 15 minutes, the sample was rinsed repeatedly with deionized water and then dried. The carbon paper was further placed in a polytetrafluoroethylene reactor containing 1 g of triphenylphosphine, and the carbon paper was taken out after reacting at 100° C. for 12 hours. Put the carbon paper into one end of the vacuum tube furnace, put 10g of urea into the other end of the vacuum tube furnace, 2 Annealing reaction at 700° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com