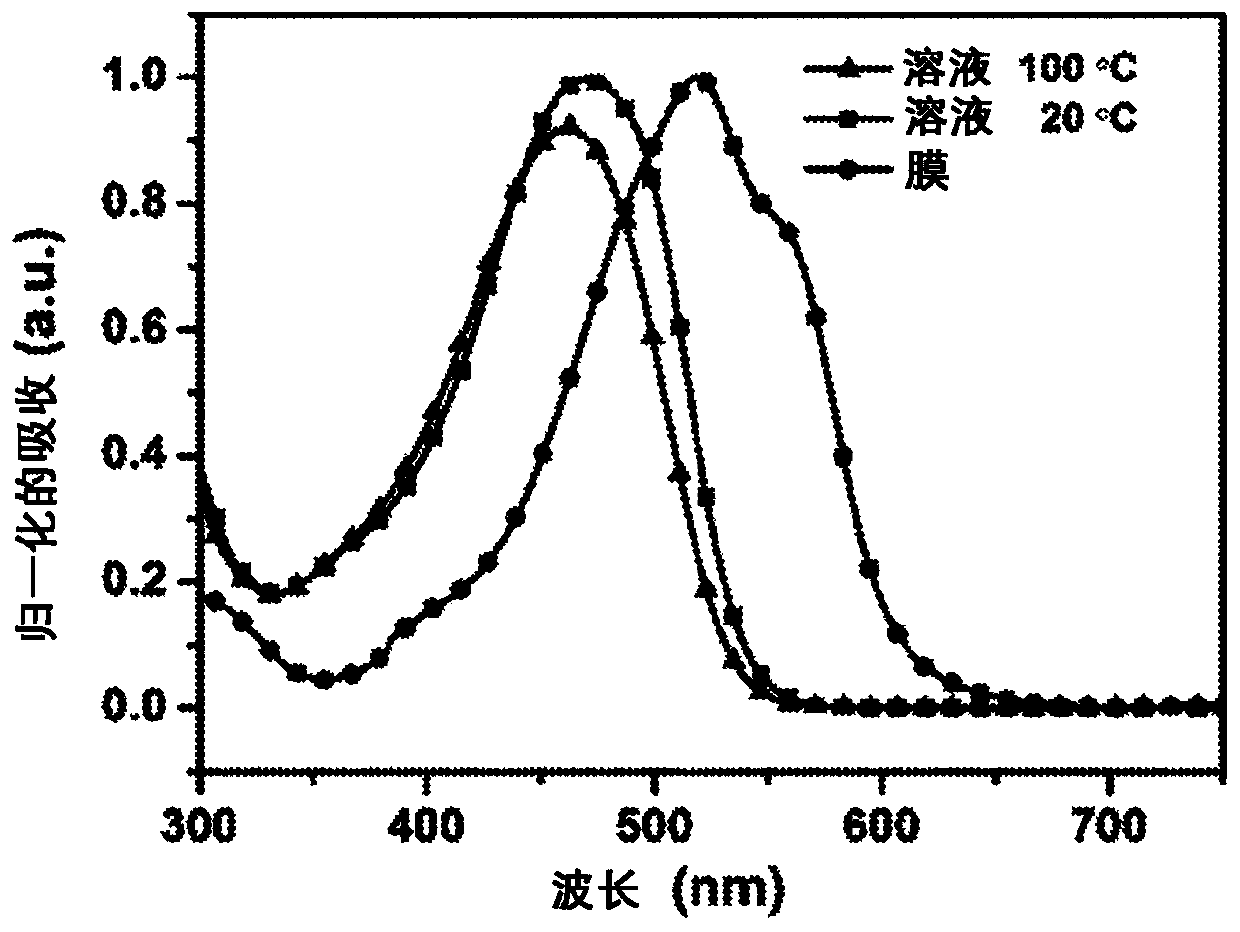
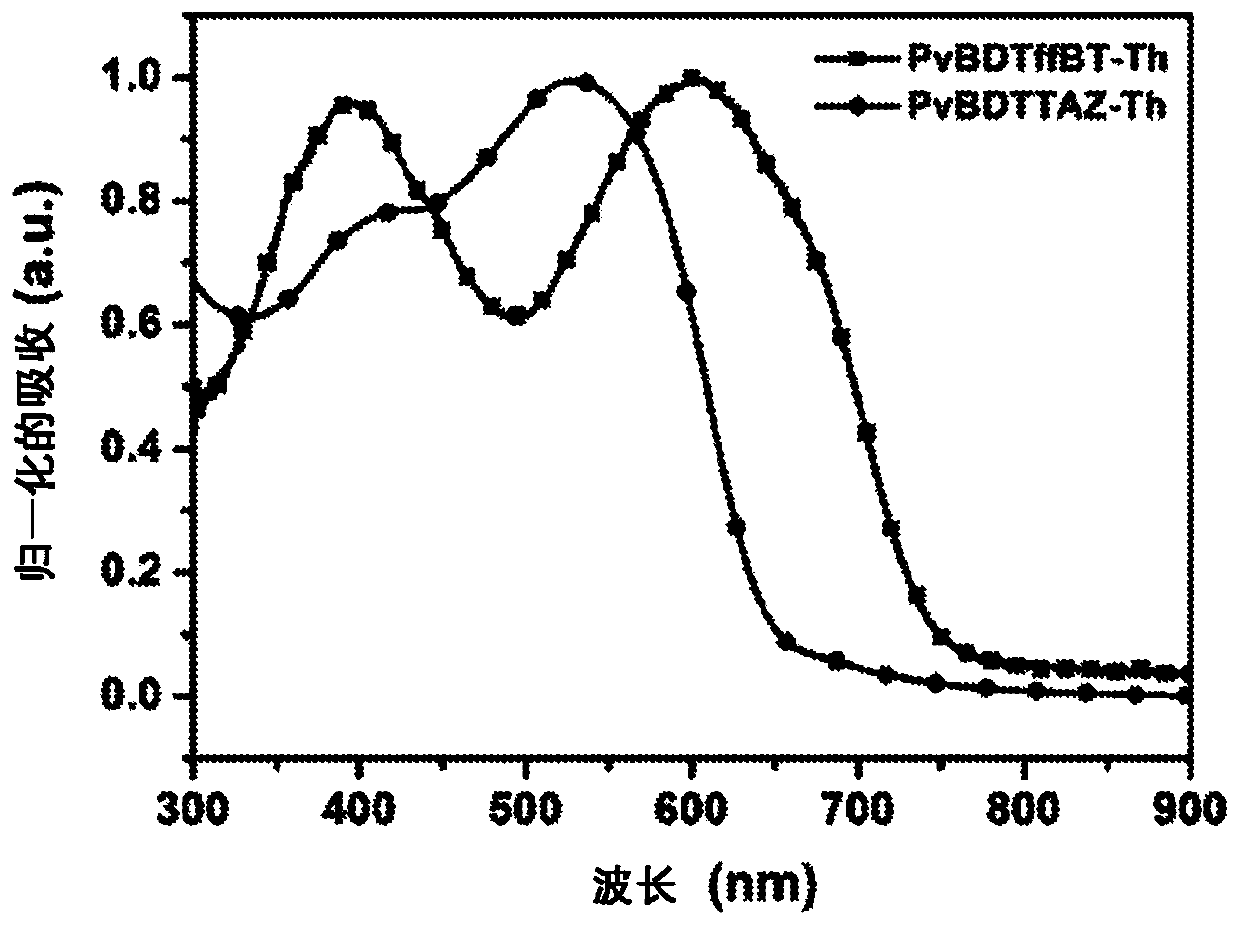
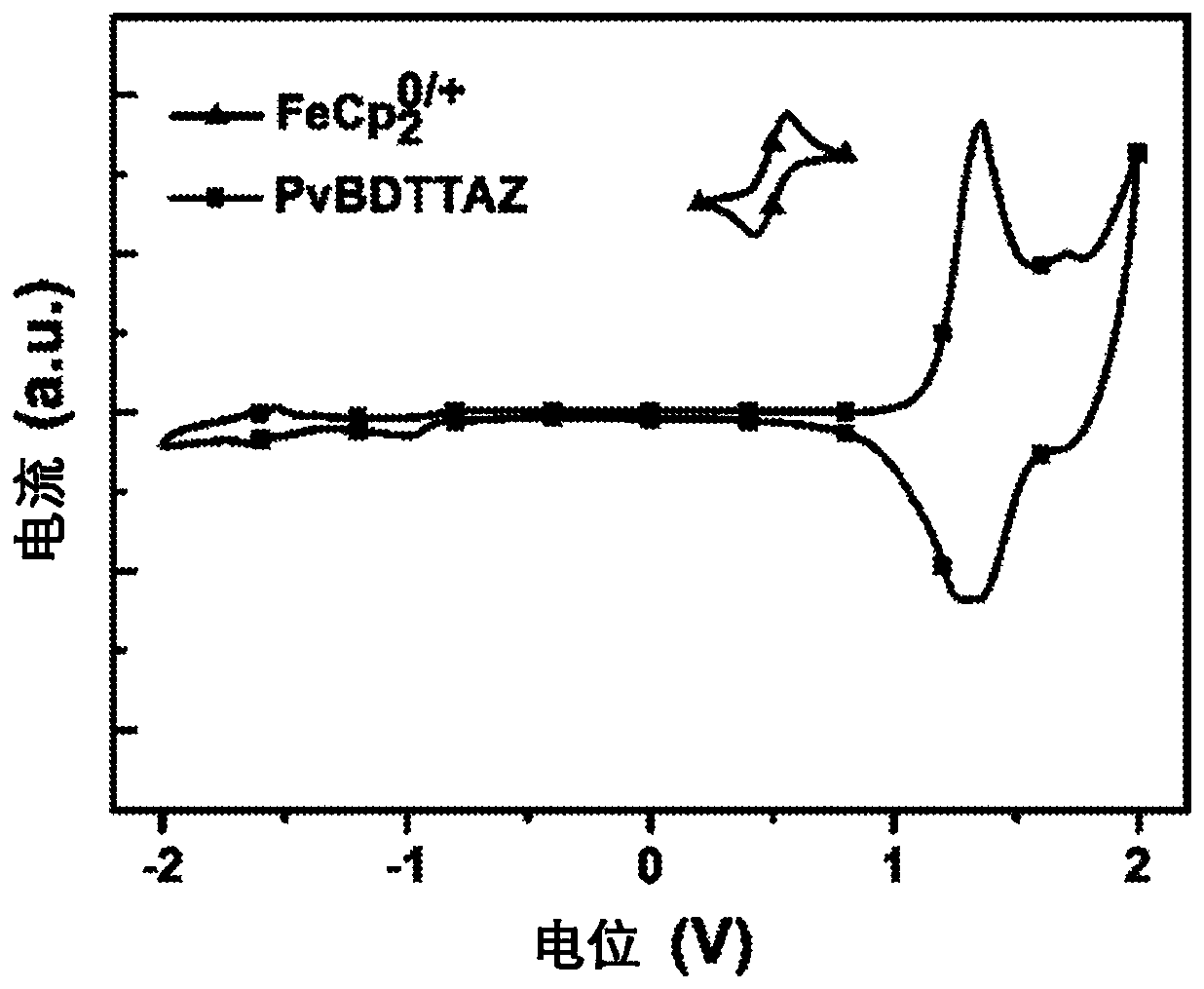
Vertical benzodithiophene-based donor-acceptor polymers for electronic and photonic applications
A conjugated polymer and acceptor technology, applied in the field of OE devices and OPV devices, can solve problems such as poor performance, and achieve the effects of wide optical band gap, high charge mobility, and strong temperature-dependent aggregation characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] Example 1: Synthesis of PvBDTTAZ
[0219]
[0220] step 1: 4,8-bis(4-(2-decyltetradecyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene (S1)。
[0221] To a solution of 3-(2-decyltetradecyl)thiophene (4.2 g, 10 mmol) in THF (40 mL) was added dropwise a solution of butyllithium (5 mL, 2.0 M in hexane) at 0°C. The solution was then stirred at 0 °C for 1 hour, after which benzo[1,2-b:4,5-b']dithiophene-4,8-dione (550 mg, 2.5 mmol) was added in one portion. The resulting yellow solution was stirred at 50 °C for 2 h before adding SnCl in 10% HCl solution (40 mL) 2 2H 2 O (11 g, 50 mmol) and the solution was stirred for another 2 hours. Hexane was added to the mixture, then, washed 3 times with water and dried over sodium sulfate. The resulting yellow oil was purified by flash chromatography to give the pure product (1.8 g, 70%) as a yellow oil.
[0222] 1 H NMR: (400MHz, CDCl 3 )7.65(d, J=5.6Hz, 2H), 7.45(s, 2H), 7.29(d, J=1.2Hz, 2H), 7.08(d, J=0.8Hz, 2H), 2.66(d,...
Embodiment 2
[0238] Example 2: Synthesis of PvBDTTAZ-Th
[0239]
[0240] ((2,6-bis(5-methylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-4,8-diyl)bis(3-(2 - decane Tetradecyl)thiophene-5,2-diyl))bis(trimethylsilane)(S5)
[0241] in N 2 S4 (860mg, 0.647mmol), tributyl (5-methylthiophen-2-yl) stannane (600.9mg, 1.55mmol), Pd 2 (dba) 3 (11mg, 0.02mmol) and P(o-tol) 3 (24 mg, 0.08 mmol) in 10 mL THF was refluxed overnight. The reaction mixture was then cooled to room temperature and the solvent was evaporated. The residue was purified by flash column chromatography (eluent: n-hexane) to obtain the product (573.8 mg, 65%) as a yellow liquid.
[0242]
[0243] 4,8-bis(5-bromo-4-(2-decyltetradecyl)thiophen-2-yl)-2,6-bis(5-methylthiophen-2-yl)benzo [1,2-b:4,5-b']dithiophene (S6)
[0244] N-Bromosuccinimide (263.5 mg, 1.48 mmol) was added to a mixture of S5 (1.01 g, 0.74 mmol) and silica gel (20 mg) in 20 mL of chloroform at 0°C. The reaction mixture was allowed to warm to roo...
Embodiment 3
[0247] Embodiment 3: the synthesis of PvBDTWBT
[0248]
[0249] The PvBDTWBT-Th polymer can be synthesized by microwave reaction or conventional reaction. In use N 2 To monomer S6 (19.7mg, 0.014mmol), S7 (9.5mg, 0.014mmol), Pd in the protected glove box 2 (dba) 3 (1.1mg, 0.002mmol) and P(o-tol) 3 (2.4 mg, 0.008 mmol) was added 0.2 mL of chlorobenzene. The reaction mixture was then sealed and heated at 140°C for 2 days (or 140°C for 2 hours for microwave reactions). The mixture was cooled to room temperature and 10 mL of toluene was added before precipitation with methanol. The solid was collected by filtration and added to the extraction thimble and sequentially washed with CH 2 Cl 2 、CHCl 3 and toluene wash. Finally the polymer was collected from toluene. The toluene solution was then concentrated by evaporation and precipitated into methanol. The solid was collected by filtration and dried in vacuo to afford the polymer as a red solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| optical band gap | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com