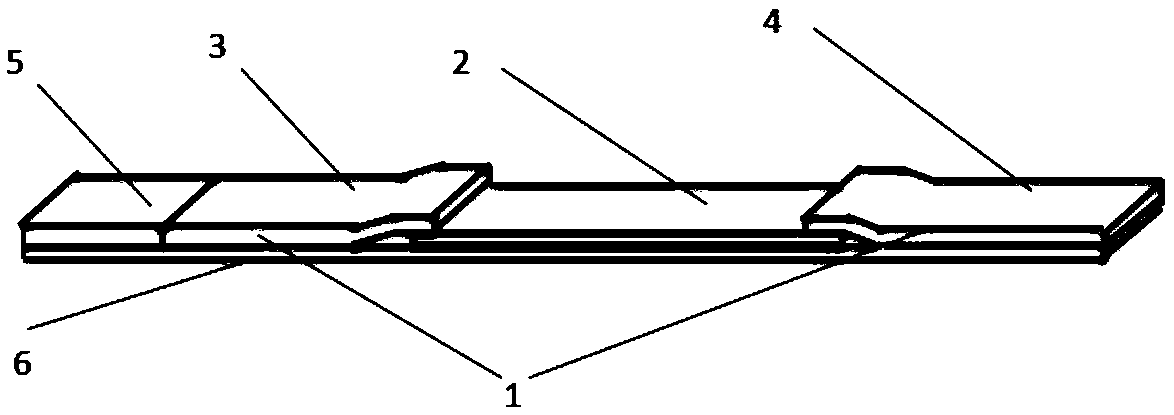
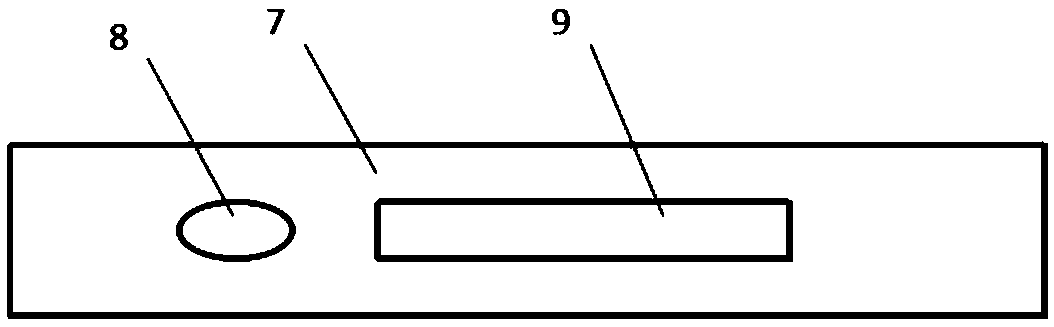
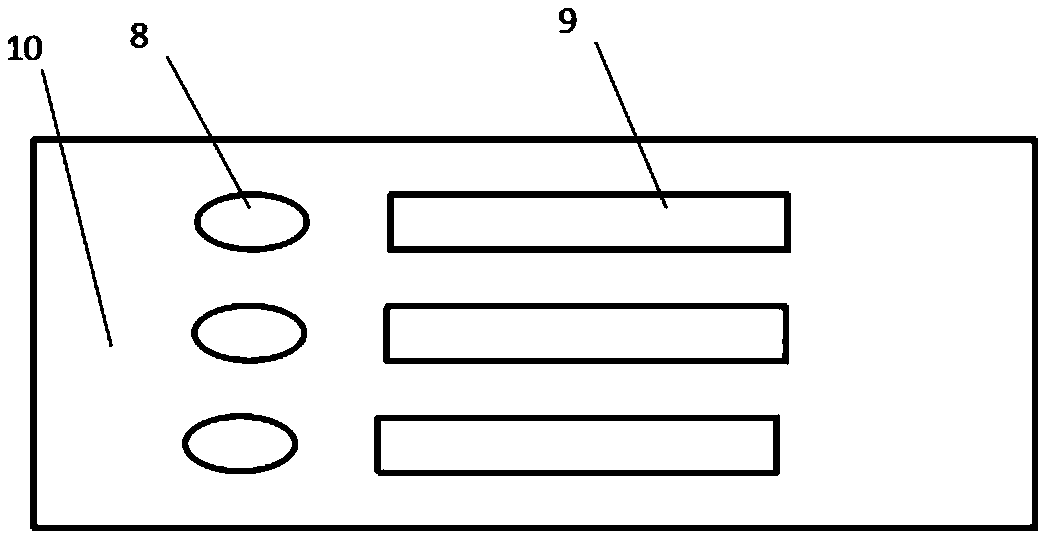
Fluorescence immunochromatography combined detection kit and preparation method thereof
A detection kit and detection card technology, which is applied in the field of clinical medical examination, can solve problems such as low accuracy, complicated test operation, and long detection time, and achieve the effects of minimizing false positives, good repeatability, and easy automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of sample pad
[0034] Use glass cellulose membrane as the sample pad, dissolve Nacl, BSA and other salt ions and proteins with buffer Tris, etc., then add a small amount of surfactant Tween20, adjust the pH to 7-8, and evenly spread it on the glass fiber according to the water absorption of the glass fiber , placed at 37 ° C and dried for 8 hours.
[0035] (2) Preparation of binding pads
[0036] Dilute the fluorescent microsphere-labeled monoclonal antibody with microsphere diluent, spread evenly on the treated sample pad, seal it after vacuum freeze-drying, and store it at room temperature. The preparation process of the fluorescent microsphere-labeled antibody is as follows:
[0037] a. Coupling of fluorescent microspheres with anti-high-sensitivity C-reactive protein monoclonal antibody
[0038]Fluorescent microspheres with a particle size of 300nm were first activated with EDC and NHS activators and stabilizers, washed after activation to remove ...
Embodiment 2
[0051] (1) Preparation of sample pad
[0052] Use glass cellulose membrane as the sample pad, dissolve Nacl, BSA and other salt ions and protein with buffer PBS, etc., then add a small amount of surfactant TritonX-100, adjust the pH to 7-8, spread evenly on the glass according to the water absorption of the glass fiber. On the fiber, dry at 37°C for 8 hours.
[0053] (2) Preparation of binding pads
[0054] Dilute the fluorescent microsphere-labeled monoclonal antibody with microsphere diluent, spread evenly on the treated sample pad, seal it after vacuum freeze-drying, and store it at room temperature. The preparation process of the fluorescent microsphere-labeled antibody is as follows:
[0055] a. Coupling of fluorescent microspheres with anti-high-sensitivity C-reactive protein monoclonal antibody
[0056] Fluorescent microspheres with a particle size of 300nm were first activated with EDC and NHS activators and stabilizers, washed after activation to remove the activato...
Embodiment 3
[0069] Detection of hypersensitive C-reactive protein, myeloperoxidase and lipoprotein phospholipase A2 fluorescent immunochromatographic detection kit.
[0070] (1) Draw a standard curve
[0071] Add different concentrations of hsCRP, myeloperoxidase, and lipoprotein phospholipase A2 antigen standards on the kit sample pad prepared according to Example 1 (7 different concentrations are taken respectively, hsCRP antigen standard Standard products were 0, 0.5, 5, 10, 40, 100, 150ng / mL, myeloperoxidase antigen standard products were 0, 10, 50, 100, 200, 500, 1000ng / mL, lipoprotein phospholipase A2 antigen The standard products were 0, 50, 100, 200, 500, 1000 and 1500ng / mL respectively, and each concentration was set in 3 repetitions), after 15 minutes, placed in a fluorescence quantitative immunochromatography instrument to obtain the fluorescence signal intensity, according to the detection line Calculate the concentration of the tested sample from the standard curve based on ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com