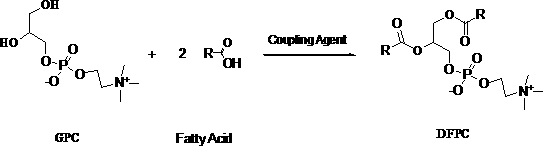
Method for preparing difatty acyl phosphatidylcholine by solid-phase reaction
A technology for fatty acyl phosphatidyl choline and glycerol phosphatidyl choline, which is applied in the field of preparation of pharmaceutical and chemical products, can solve the problems of high cost and unfavorable industrialization, and achieves the effects of low cost, realization of industrialized production and short production cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 10 g of glycerol phosphatidylcholine and 20 mL of methanol into a 250 mL three-necked flask, and dissolve under stirring at room temperature. Add 20 g of alumina, continue to stir for 1 h, and recover the methanol solvent by distillation under reduced pressure, and vacuum-dry the obtained solid-phase loading mixture for future use.
[0026] Add 160mL of dichloromethane and 55g of stearic acid into a 500mL three-neck flask, heat up to reflux with stirring until the stearic acid dissolves, slowly add 40g of N,N'-dicyclohexylcarbodiimide (DCC), and continue to reflux after the addition is complete 2h. 30 g of alumina-loaded glycerol phosphatidylcholine mixture was added to the above reaction solution, and TLC was followed until the end of the reaction. Alumina was recovered by filtration, dichloromethane was recovered by spin-drying, and the crude solid obtained was beaten with 200 mL of acetone at room temperature to remove impurities. After beating three times, 20....
Embodiment 2
[0028] Add 50 g of glycerol phosphatidylcholine and 100 mL of ethanol into a 500 mL three-necked flask, and dissolve under stirring at 50°C. 50 g of silica gel was added, stirring was continued for 1 h, the ethanol solvent was recovered by distillation under reduced pressure, and the obtained solid-phase loading mixture was vacuum-dried for future use.
[0029] Add 800mL of n-heptane and 275g of oleic acid into a 1000mL three-neck flask, heat up to reflux with stirring until the oleic acid dissolves, slowly add 155g of N,N'-carbonyldiimidazole (CDI), and continue to reflux for 2 hours after the addition is complete. Add 100 g of silica gel-loaded glycerol phosphatidylcholine mixture to the above reaction solution, and track it by TLC until the end of the reaction. Silica gel was recovered by filtration, n-heptane was recovered by spin-drying, and the crude solid obtained was beaten with 2000 mL of methyl tert-butyl ether at room temperature to remove impurities. After beating...
Embodiment 3
[0031] Add 10 g of glycerol phosphatidylcholine and 40 mL of isopropanol into a 250 mL three-necked flask, and dissolve under stirring at room temperature. Add 30 g of alumina, continue to stir for 1 h, and recover the isopropanol solvent by distillation under reduced pressure, and vacuum-dry the obtained solid-phase loaded mixture for future use.
[0032] Add 160mL of chloroform and 40g of stearic acid into a 500mL three-neck flask, heat up to reflux with stirring until the stearic acid dissolves, and slowly add 32g of N,N'-dicyclohexylcarbodiimide (DCC). Add 40 g of the alumina-supported glycerol phosphatidylcholine mixture into the above reaction solution, and track the reaction until the end of the reaction by TLC. Alumina was recovered by filtration, chloroform was recovered by spin-drying, and the crude solid obtained was beaten with 300 mL of methyl isobutyl ketone at room temperature to remove impurities. After beating three times, 20.4 g of high-purity distearoylphos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com