Attenuated virus vector system, application of attenuated virus vector system in preparation of anti-malignant tumor drugs and drug use method
A technology of attenuated virus and carrier system, which is applied in the field of biomedicine and can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment provides an attenuated virus vector system, the virus is an RNA virus, the genetic material of the virus does not have the ability to integrate in mammalian cells, the virus is Rhabdoviridae, vesicular stomatitis virus, the virus Molecules encoded by chimeric structural genes of the carrier system include at least one specific inhibitor for immune checkpoint molecules.
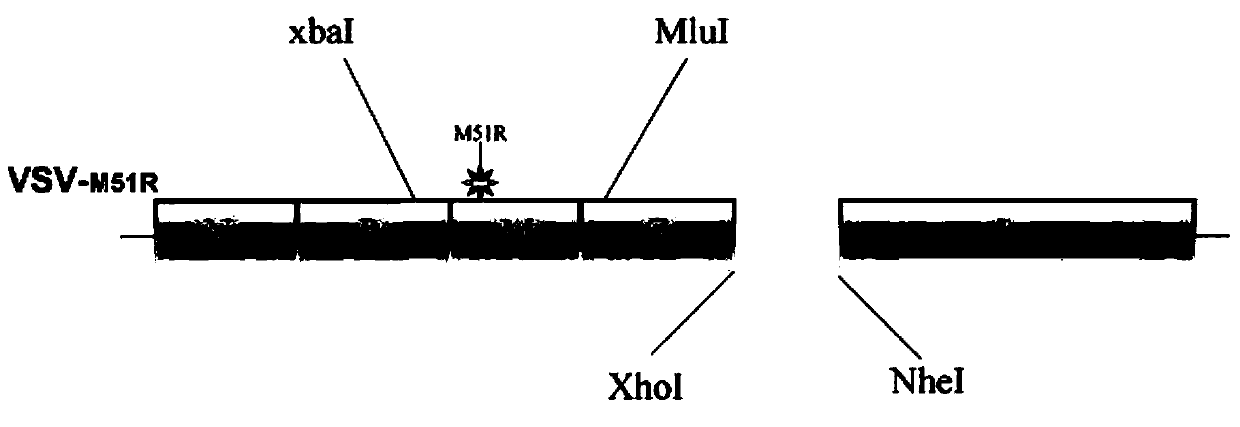
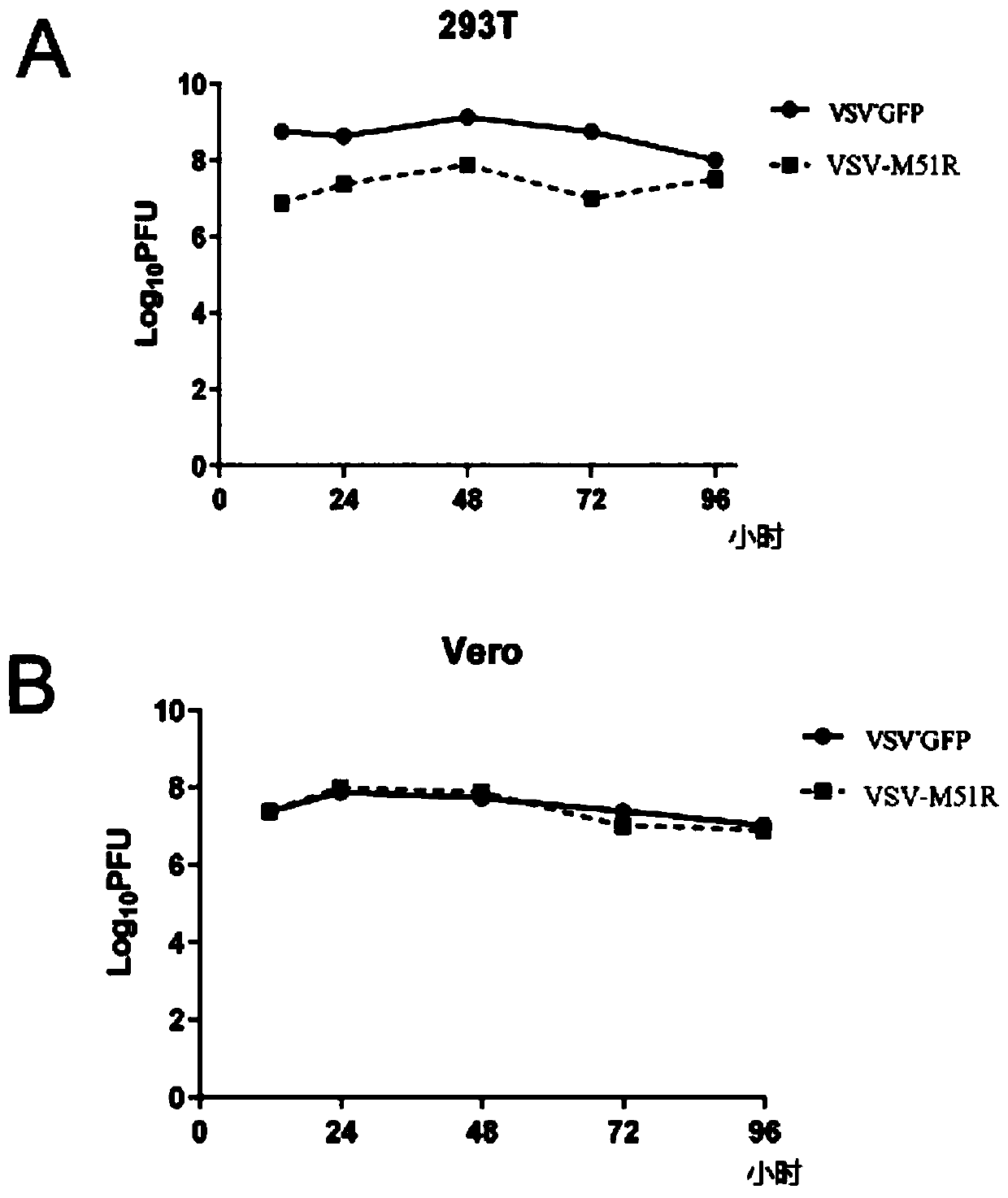
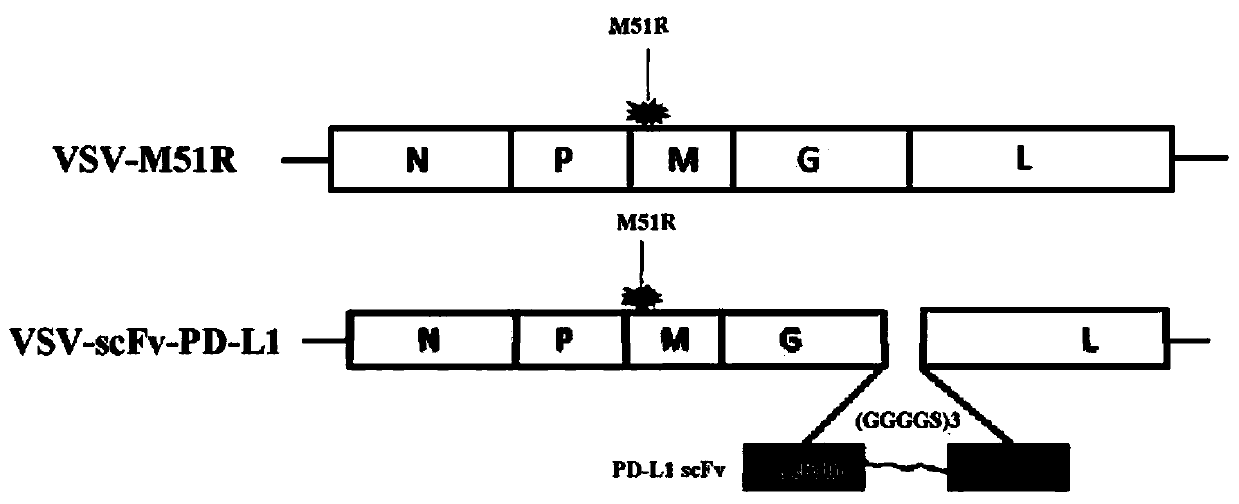
[0049] Preferably, the vesicular stomatitis virus is an attenuated mutant strain, wherein the 51st amino acid of the M gene undergoes a M->R mutation, and the point mutation includes at least one or more point mutations in the M gene of the virus.
[0050] Preferably, antibodies and antibody analogs of immune checkpoint molecules on the cell surface are one or more of single-chain antibodies, single-domain antibodies, bifunctional single-chain antibodies or multifunctional single-domain antibodies, and the antibodies and antibody analogs The immune checkpoint molecules are abnormally exp...
Embodiment 2
[0061] This embodiment provides an application of an attenuated virus vector system in the preparation of anti-cancer drugs, which includes the above-mentioned attenuated virus vector system.
[0062] Preferably, the active substance in the medicine also includes one or more of clofibrate, choline, methionine, nicotinic acid or ursodeoxycholic acid.
[0063] Preferably, the drug also includes one or more oncolytic viruses or corresponding attenuated strains of vaccinia virus, herpes virus, measles virus, Newcastle disease virus, alphavirus, parvovirus and enterovirus strains.
[0064] In yet another aspect, this embodiment also provides an application of an attenuated virus vector system in drugs for killing abnormally proliferative cells, inducing and promoting anti-tumor immune responses, or eliminating immunosuppression in tumor tissue microenvironments, and the drugs are at least Said attenuated viral vector system is included.
[0065] Preferably, the abnormal proliferat...
Embodiment example 3
[0082] Example 3: In this example, the treatment of rectal cancer in mice by oncolytic virus VSV-scFv-PD-L1 is described in detail.
[0083] The first is the establishment of a mouse colorectal cancer model, such as Figure 7 As shown in A, each CB7BL / 6 was subcutaneously inoculated with 2.0*10^5 (200uL) MC38 colon cancer cells. The tumor size was measured every other day and calculated according to the following formula: M12*M2 / 2 (M1: short diameter, M2: long diameter). After the tumor volume of mice in each group grew more than 50mm3, 107PFU (20ul) intratumoral injection of virus was given on day7, day9 and day11 respectively, and the changes in tumor volume were continuously observed and recorded.
[0084] Pharmacodynamic performance testing of VSV-scFV-PDL1 immunotherapy, such as Figure 7 As shown in B, similar to non-small cell lung cancer in mice, oncolytic virus-loaded PD-L1 single-chain antibody treatment, compared with control virus VSV-M51R treatment, can effectiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com