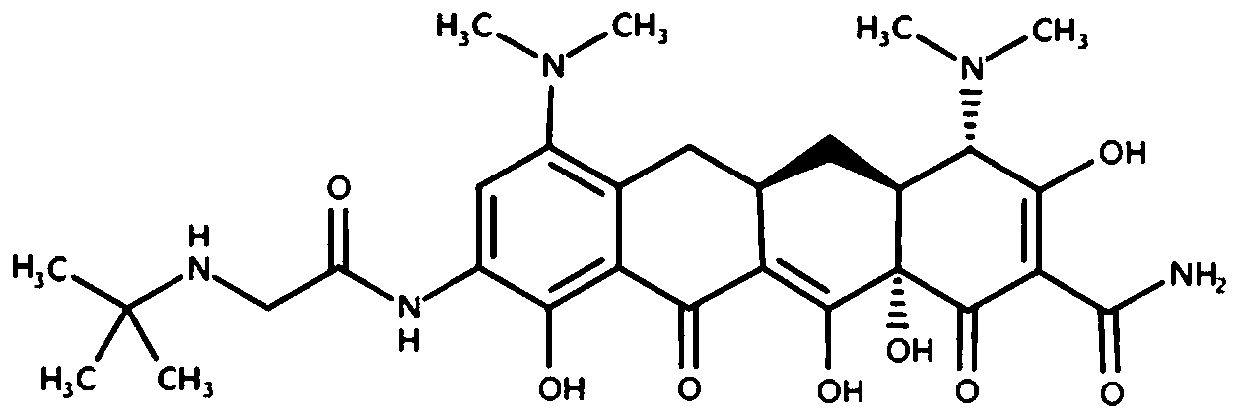
Tigecycline liposome preparation
A technology of liposome preparation and tigecycline, applied in the directions of liposome delivery, tetracycline active ingredients, inorganic non-active ingredients, etc., can solve the problems of low local concentration, oxidative degradation, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The detection method of embodiment 1 tigecycline
[0061] Establish tigecycline standard curve in the present embodiment, concrete process is as follows:
[0062] Using ultraviolet (ultraviolet, UV) detection and analysis method: the detection instrument is TECAN 200 PRO; the detection wavelength is 350nm; the detection temperature is 23°C; the detection orifice plate is 96 well plates, UV-transparent; assay volume 200 μL.
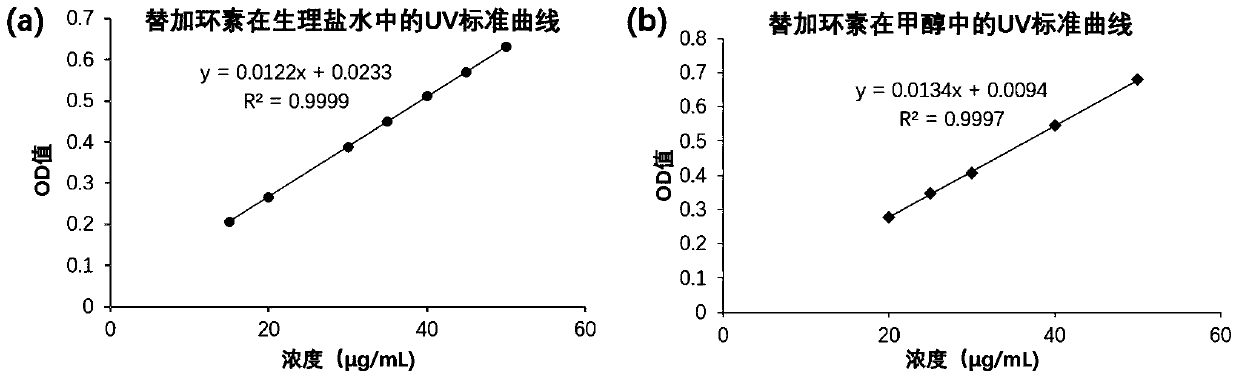
[0063] 1.1 UV standard curve of tigecycline in normal saline
[0064] Accurately weigh 2.51mg of tigecycline, dissolve it in a 25mL volumetric flask with normal saline, and obtain a tigecycline aqueous solution with a concentration of 0.10mg / mL; use normal saline to carry out gradient dilution of tigecycline solution to obtain Tigecycline standard solutions with concentrations of 15 μg / mL, 20 μg / mL, 30 μg / mL, 35 μg / mL, 40 μg / mL, 45 μg / mL and 50 μg / mL. The standard solution of tigecycline with the above concentration was detected by UV method, ...
Embodiment 2
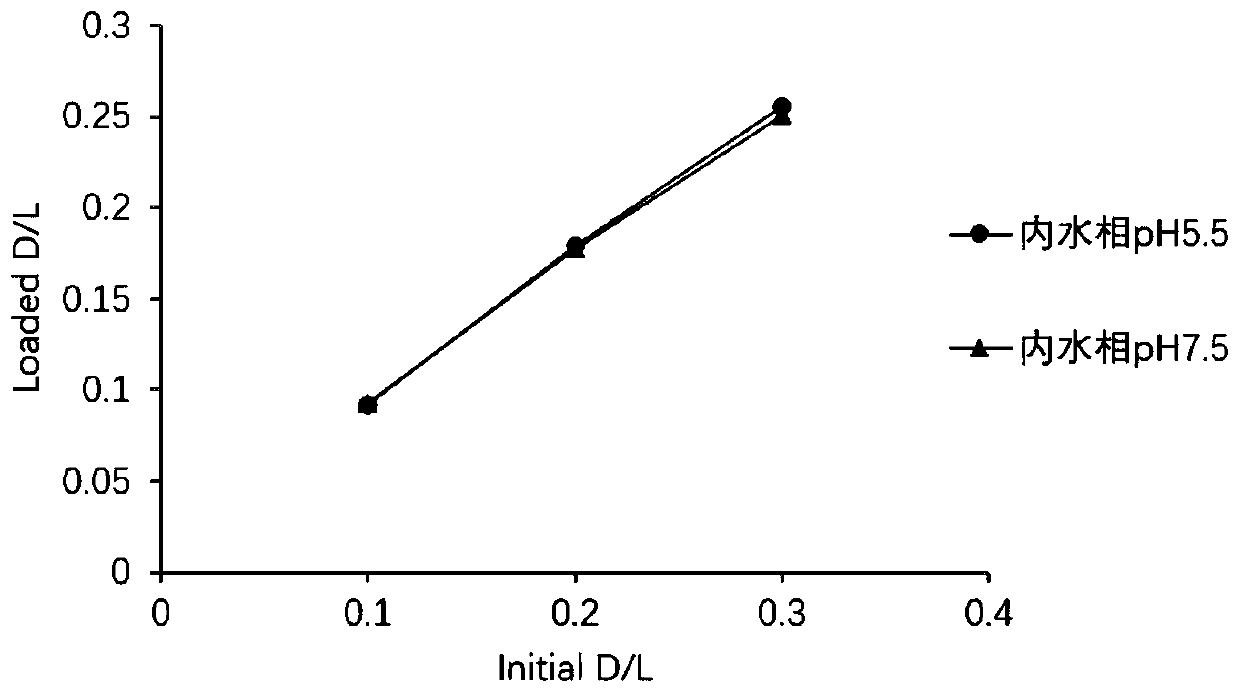
[0073] Example 2 Preparation and Characterization of Tigecycline Liposomes Using Calcium Acetate Solution with pH 4.0~7.0 as Internal Water Phase
[0074] All the phospholipids used in this example were purchased from Lipoid Company of Germany. Specifically hydrogenated soy lecithin (HSPC, molecular weight 783.8); PEGylated phospholipid is specifically distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000); cholesterol (CHOL, molecular weight 386.7); Cyclocycline was purchased from TCI Company; the calcium acetate used was purchased from Shanghai Bailingwei Chemical Technology (J&K SCIENTIFIC) Co., Ltd.; the acetic acid used was purchased from Sinopharm Chemical Reagent Co., Ltd.
[0075] It should be noted that the phospholipids used in the following examples are all hydrogenated soy lecithins of Lipoid Company; the pegylated phospholipids used are all distearoylphosphatidylethanolamine-polyethylene glycol of Lipoid Company; The cholesterol used was Lipoi...
Embodiment 3
[0095] Example 3 Preparation and Characterization of Tigecycline Liposomes Using Calcium Acetate Solution with pH 7.0~8.0 as Internal Water Phase
[0096] Sodium hydroxide used in this example was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd.
[0097] Accurately weigh 1.5860g CaAc 2 ·H 2 O, add an appropriate amount of deionized water, adjust the pH to 7.5 / 8.0 with 1M acetic acid solution or 1M sodium hydroxide solution, add deionized water to set the volume to 30mL, and obtain a 300mM calcium acetate buffer solution with a pH of 7.5 / 8.0.
[0098] To prepare tigecycline liposomes, refer to Example 2 for the specific preparation process. The difference is that in step (3), 10ml of 300mM calcium acetate buffer solution with a pH of 7.5 / 8.0 should be added to the ethanol mixed solution obtained in step (1), instead of acetic acid with a pH of 4.0 / 5.5 / 7.0 calcium buffer.
[0099] The characterization method of tigecycline liposome is with reference to embodiment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com