Synthesis method of m-dichlorobenzene
A synthesis method and technology of m-dichlorobenzene, which are applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of low selectivity, large equipment corrosion and high equipment requirements , to achieve the effect of mild reaction conditions, less three wastes, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of Aluminum Trichloride-Chlorobutylmethylimidazolium Ionic Liquid
[0026] Put 20g (0.115mol) of 1-methyl-3-butylimidazole chloride in the flask, and slowly add 46g (0.345mol) of AlCl after raising the temperature to 50°C under the protection of nitrogen 3 , start stirring while adding, continue to keep warm and stir for 10 hours after the addition, and then put it into a vacuum drying oven to dry for later use.
[0027] (2) Preparation of m-dichlorobenzene
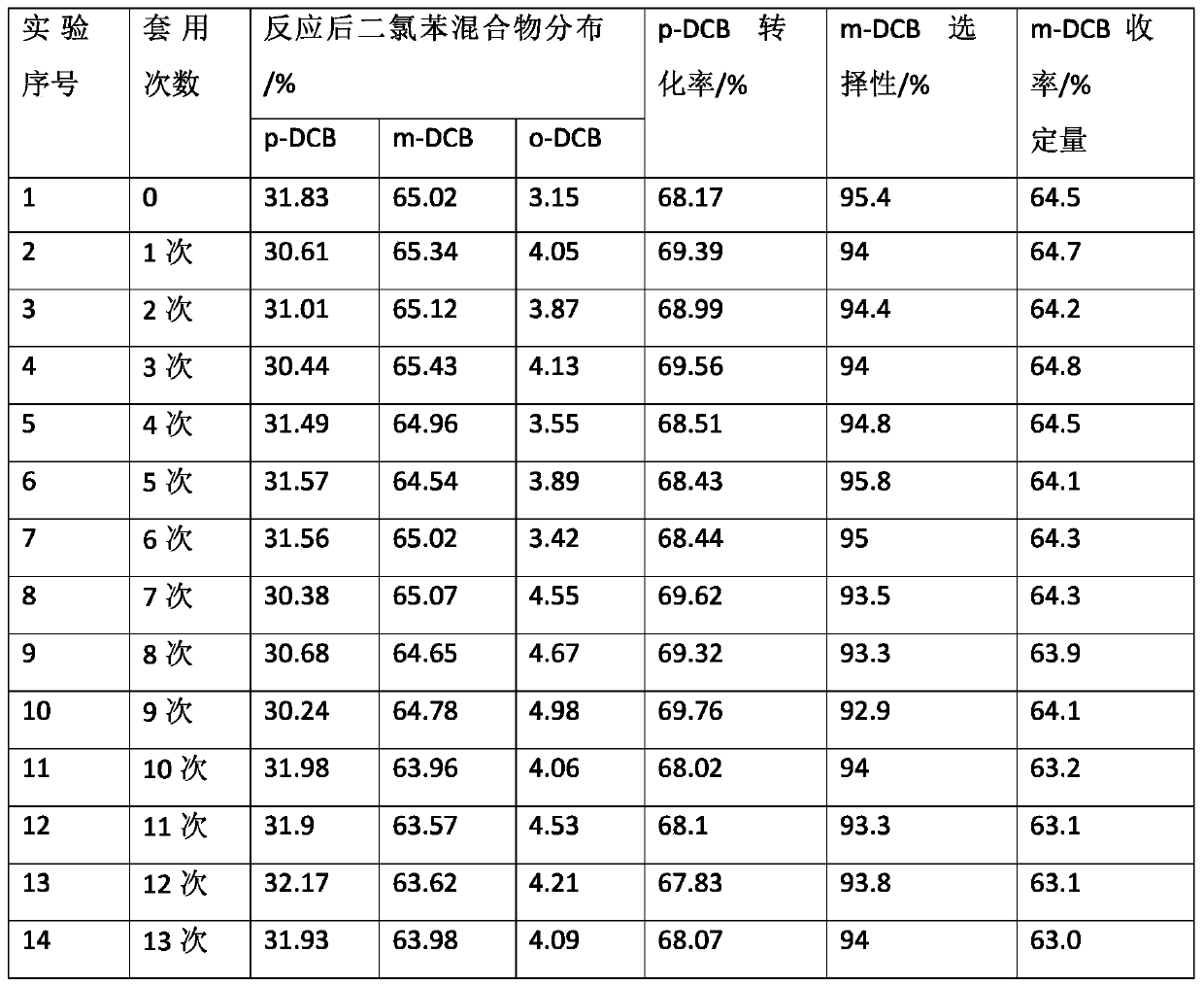
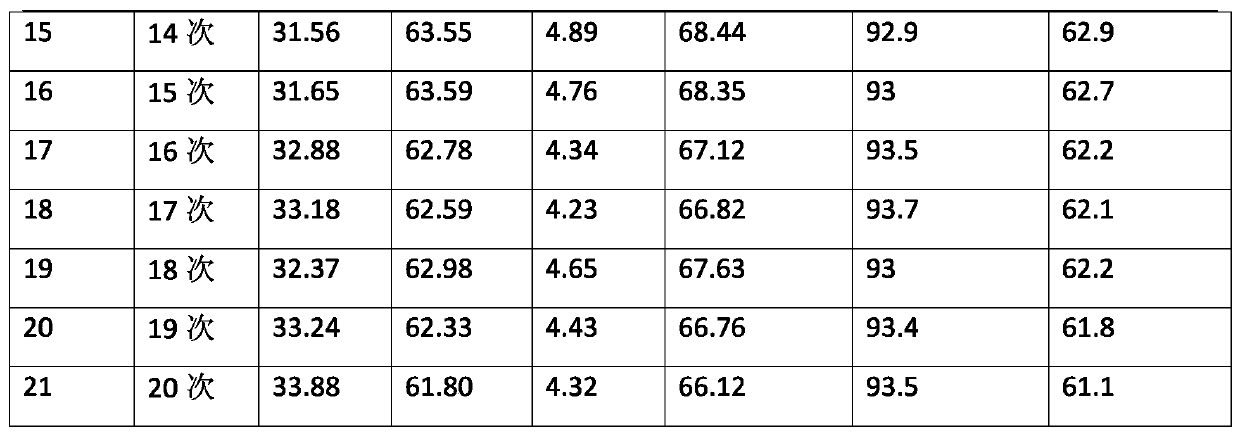
[0028] Put 50.7g (0.345mol) of pre-melted p-dichlorobenzene in the flask, start stirring and slowly add 7.5g of the catalyst prepared in step (1), react at 170°C for 4h under the protection of an inert gas, cool down to room temperature, and separate the liquids Separating the lower floor catalyst, the upper organic phase adopts gas chromatography analysis, and calculates the reaction product with the area normalization method to form: p-dichlorobenzene is 31.83%, m-dichlorobenzene is 65.02%, ortho...
Embodiment 2
[0036] (1) Preparation of ferric chloride-chlorobutylpyridine ionic liquid
[0037] Put 10g (0.059mol) chloro-N-butylpyridine in the flask, raise the temperature to 80°C under the protection of nitrogen, then slowly add 18.9g (0.117mol) FeCl 3 , start stirring while adding, continue to keep warm and stir for 12 hours after the addition, and then put it into a vacuum drying oven to dry for later use.
[0038] (2) Preparation of m-dichlorobenzene
[0039] Put 40g (0.272mol) of o-dichlorobenzene in a flask, start stirring and slowly add 4g of the catalyst prepared in step (1), react at 160°C for 6h under the protection of an inert gas, cool down to room temperature, and separate the catalyst from the lower layer. The upper organic phase is analyzed by gas chromatography, and the area normalization method is used to calculate the reaction product to form: o-dichlorobenzene is 32.23%, m-dichlorobenzene is 61.02%, p-dichlorobenzene is 6.75%, and the conversion rate is 67.77%. sex ...
Embodiment 3
[0041] (1) Preparation of zinc chloride-tetramethylammonium chloride ionic liquid
[0042] Put 15g (0.137mol) of tetramethylammonium chloride in the flask, and slowly add 46.6g (0.342mol) of ZnCl after heating up to 60°C under the protection of nitrogen 2 , start stirring while adding, continue to keep warm and stir for 8 hours after the addition, and then put it into a vacuum drying oven to dry for later use.
[0043] (2) Preparation of m-dichlorobenzene
[0044] Put 45g (0.306mol) of a mixture of o-dichlorobenzene and p-dichlorobenzene (p-dichlorobenzene accounts for 36%, o-dichlorobenzene accounts for 64%) in the flask, start stirring and slowly add 5g of the mixture prepared in step (1). The catalyst was reacted at 180°C for 3 hours under the protection of an inert gas, cooled to room temperature, and the lower catalyst was separated by liquid separation. The upper organic phase was analyzed by gas chromatography, and the composition of the reaction product was calculated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com