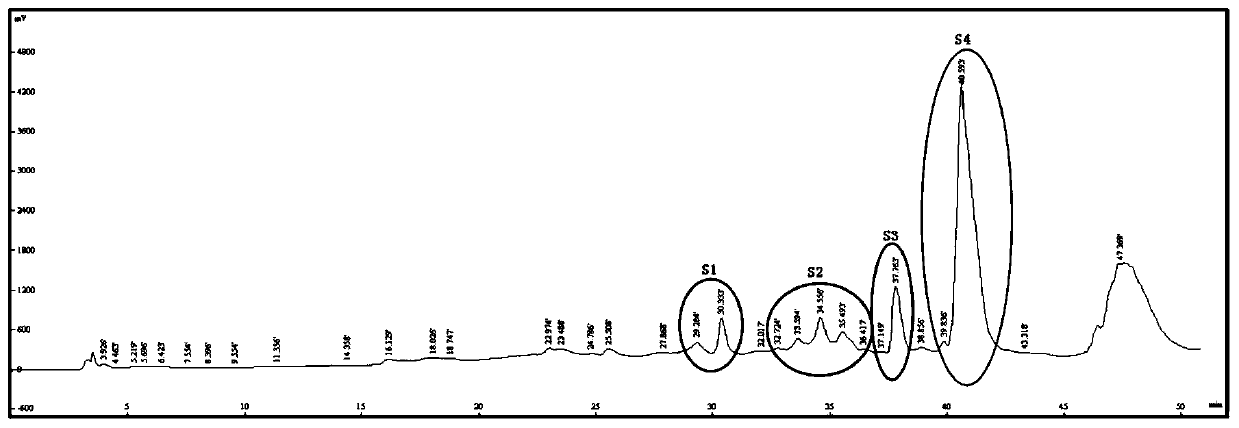
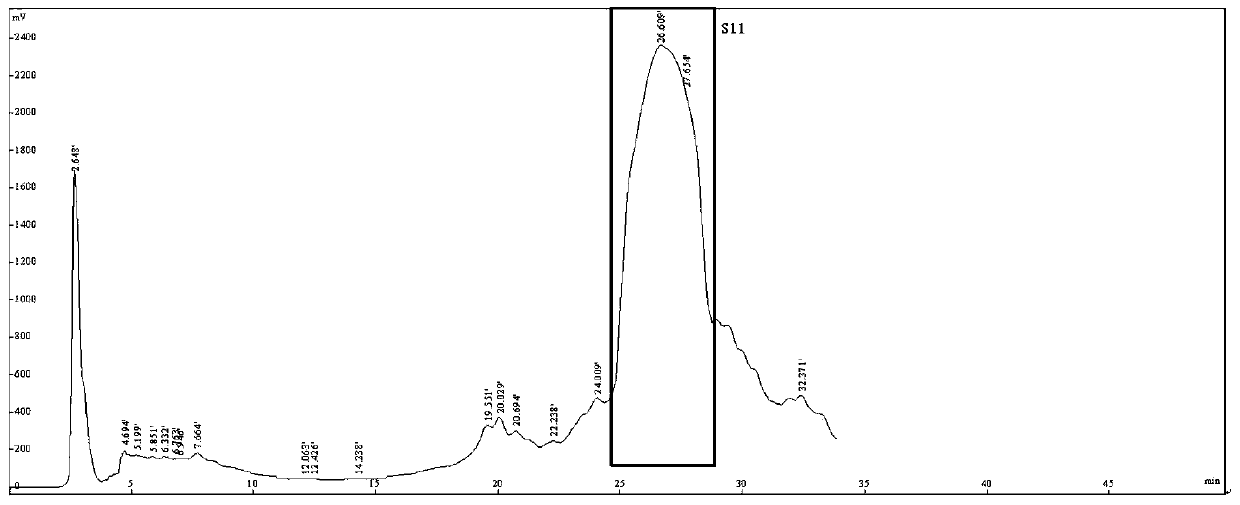
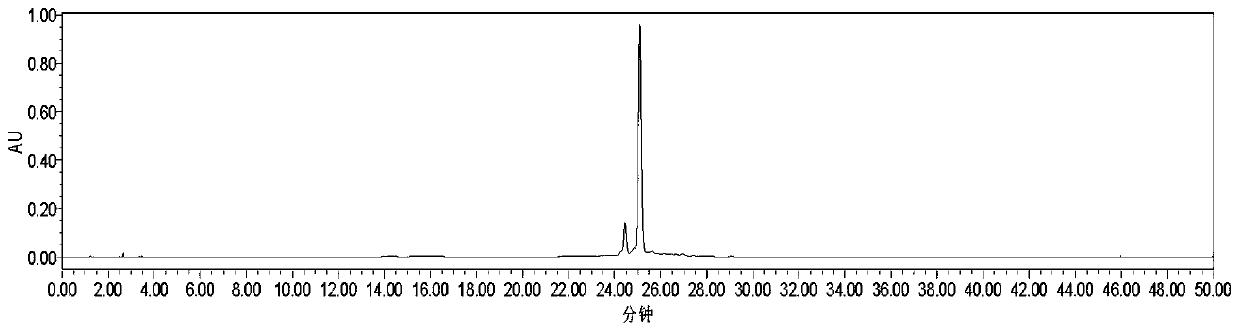
Novel stilbene compound oligomers in sword iris (Iris lacteal Pall .var .chinensis(Fisch .)Koidz .) seed kernels, and extraction method and usage thereof
A technology of stilbene compounds and compounds, applied in the field of new oligomeric stilbene compounds and their extraction, can solve problems such as side effects of patients, and achieve the effects of expanding clinical applications, inhibiting lipid production, and inhibiting lipid droplet accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation of embodiment 1 oligomeric stilbene class novel compound
[0084] (1) washing the seeds of Rhizoma rhizome after removing impurities with pure water, drying in the shade, peeling, and pulverizing to 30 orders to obtain the raw material of seed kernels of Rhizoma rhizome;
[0085] (2) 22kg of Sizum officinalis seeds were soaked and extracted at room temperature for 12h with 5% NaOH, wherein the 5% NaOH solution was 3kgNaOH dissolved in 60L of ultrapure water, and the extract was collected by filtration;
[0086] (3) Add dilute H to the extract obtained in step (2) 2 SO 4 solution, adjusted to PH=3, and allowed to stand at room temperature for 1 hour to cause precipitation;
[0087] (4) Collect the precipitate produced in step (3) and centrifuge at room temperature, the centrifugation condition is: 4500rpm, 10min, collect the precipitate obtained;
[0088] (5) Dissolve the acid precipitate collected in step (4) with 12L of 95% ethanol to fully dissolve ...
Embodiment 2
[0100] The preparation of embodiment 2 oligomeric stilbene class novel compounds
[0101] (1) washing the seeds of Rhizoma rhizome after removing impurities with pure water, drying in the shade, peeling, and pulverizing to 30 orders to obtain the raw material of seed kernels of Rhizoma rhizome;
[0102] (2) Soak 22kg of Sizum officinalis seed kernels in 10% NaOH at a normal temperature for 8 hours, wherein the 10% NaOH solution is 6kg NaOH dissolved in 60L of ultrapure water, and the extract is collected by filtration;
[0103] (3) Add hydrochloric acid dropwise to the extract obtained in step (2), adjust the pH to 3, and let it stand at room temperature for 1 hour to cause precipitation;
[0104] (4) Collect the precipitate produced in step (3) and centrifuge at room temperature, the centrifugation condition is: 4500rpm, 10min, collect the precipitate obtained;
[0105] (5) Dissolve the acid precipitate collected in step (4) with 12L of methanol to fully dissolve it;
[010...
Embodiment 3
[0111] The preparation of embodiment 3 oligomeric stilbene class novel compounds
[0112] (1) washing the seeds of Rhizoma rhizome after removing impurities with pure water, drying in the shade, peeling, and pulverizing to 30 orders to obtain the raw material of seed kernels of Rhizoma rhizome;
[0113] (2) 22kg of Sizum officinalis seeds were soaked and extracted at room temperature with 1% NaOH for 18h, wherein the 1% NaOH solution was 0.6kgNaOH dissolved in 60L of ultrapure water, and the extract was collected by filtration;
[0114] (3) Add dilute H to the extract obtained in step (2) 2 SO 4 solution, adjusted to PH=3, and allowed to stand at room temperature for 1 hour to cause precipitation;
[0115] (4) Collect the precipitate produced in step (3) and centrifuge at room temperature, the centrifugation condition is: 4500rpm, 10min, collect the precipitate obtained;
[0116] (5) Dissolve the acid precipitate collected in step (4) with 12L of 95% ethanol to fully dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com