Detection material for anti-myelin oligodendrocyte glycoprotein (MOG) autoantibody in human body fluid, preparation method and application
A technology of autoantibodies and detection materials, applied in the field of biomedicine, can solve the problems of large demand for antibodies, long detection time, low sensitivity, etc., and achieve the effect of strong operation skills, short detection cycle and high detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
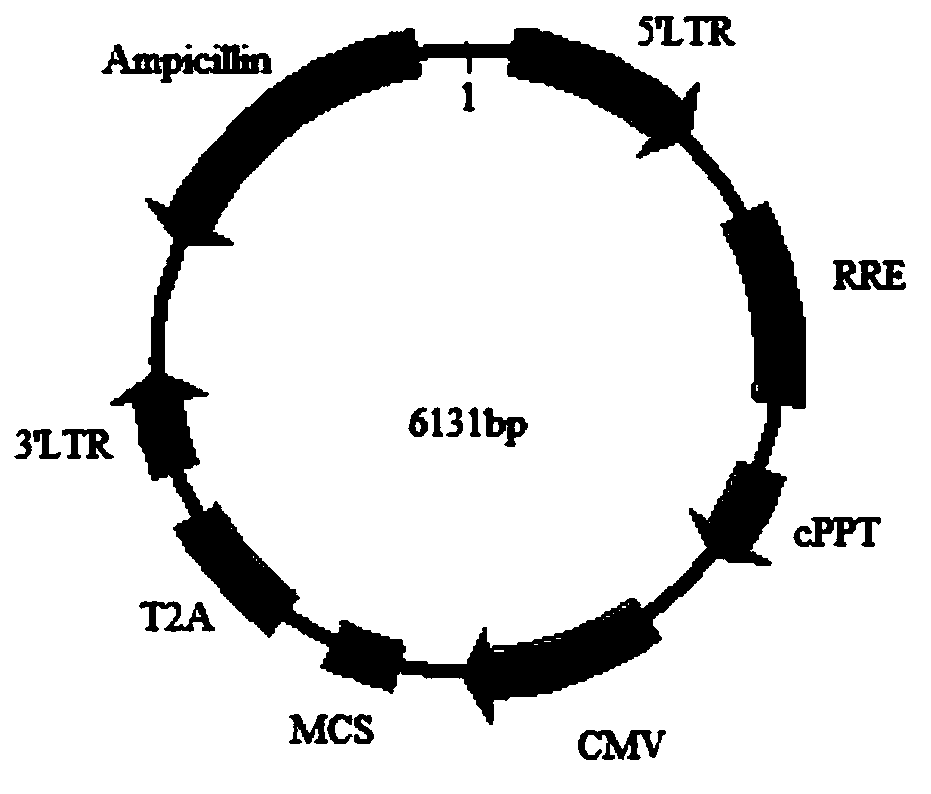
[0041] Step 1, plasmid construction: Obtain the CDS sequence of MOG as the target gene, insert the target gene with restriction sites into the 17T2A plasmid vector to obtain the recombinant plasmid vector 17T2A-MOG, extract the plasmid for subsequent experiments after the sequencing is correct, this implementation The plasmid vector construction of example specifically comprises the following steps:
[0042] Step 1.1: Obtain the CDS sequence of MOG as the target gene by PCR method (artificial synthesis method is also optional), and add SalI / NotI restriction sites at both ends of the target gene;
[0043] Step 1.2: Insert the target gene with the restriction site into the 17T2A plasmid vector, the insertion site is SalI / NotI, to obtain the recombinant vector, which is named 17T2A-MOG;
[0044] Among them, the 17T2A plasmid vector deletes the copGFP element on the basis of the pCDH-CMV-MCS-EF1-copGFP vector, and replaces the FE1 promoter with the T2A element at the same time. Th...
Embodiment 2
[0075] This embodiment discloses a method for preparing a sealing material, which specifically includes:
[0076] 1) Cut the carrier film (glass fiber mat in this example) into small square pieces of 0.5cm×0.5cm, put 15μL of a mixture of 1.9M sucrose and 1.9M glucose on each small piece of glass fiber mat, and bake at 100°C for 20min. Store at room temperature for later use;
[0077] 2) Extraction of 17T2A cell (PS) protein: scrape the 17T2A cells obtained in step 2.3 of the above example with a scraper, collect the 17T2A cells in a 15ml centrifuge tube, 1000rpm, 5min, room temperature, discard the supernatant. Add 1mL of 1×PBS (or normal saline) to the precipitate, pipette to mix, transfer the liquid to a 1.5ml EP tube, 700g, 5min, room temperature, discard the supernatant. Add 1 / 2 volume of the cell pellet volume blocking protein extract (1.25% sodium deoxycholate, 0.25% Triton X-100, 0.75% CHAPS, 20mMNaCl, 2×PI), pipette to mix and transfer the liquid to a 2ml EP tube , v...
Embodiment 3
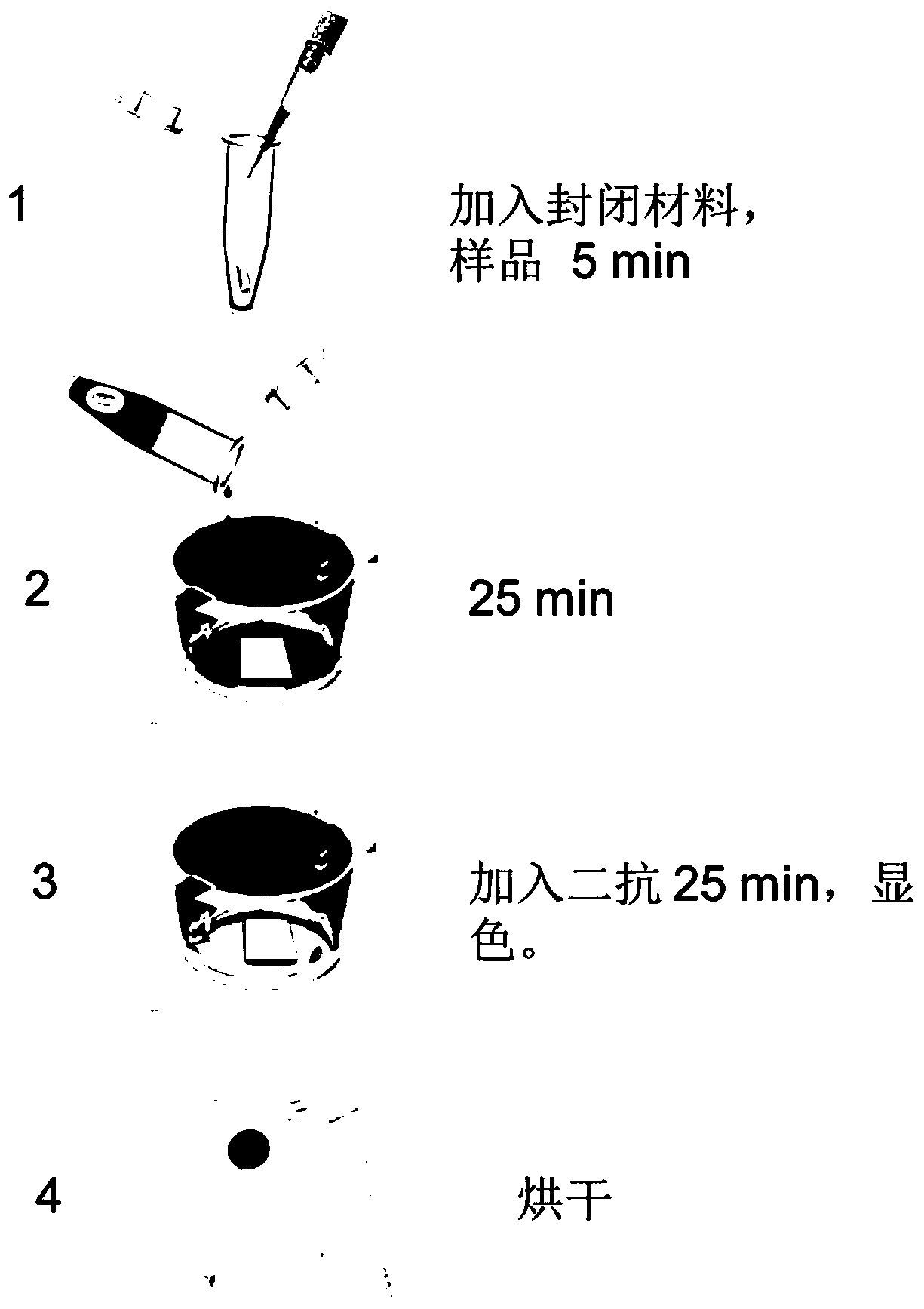
[0081] The detection material for anti-MOG autoantibodies in human body fluid prepared in Example 1 is used to detect MOG antibodies in samples. Specifically, the detection process for detecting MOG antibodies in samples includes:
[0082] 1) Place the membrane protein-cell membrane complex detection material obtained in step 4 of the above example in a 24-well plate, with the membrane protein antigen-coated side facing up.
[0083] 2) Serum blocking: Dilute the sample to be tested at 1:250 with working solution (1×PBS, 0.5% TritonX-100, 0.04% EDTA), add a piece of blocking material prepared in Example 4 to every 250 μL of diluted serum, and keep at room temperature After standing for 2 minutes, vortex for a few seconds, and then stand at room temperature for 5 minutes.
[0084] 3) Serum incubation: Add the blocked serum to a 24-well plate containing membrane protein-cell membrane complex detection material, 250 μL / well, place the 24-well plate on a horizontal shaker (frequenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com