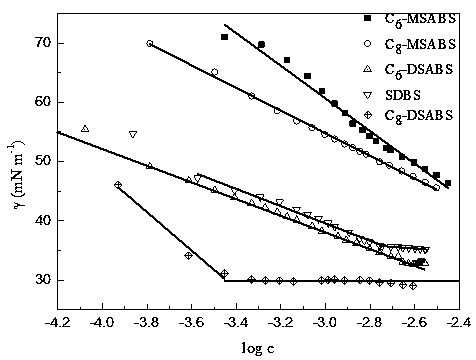
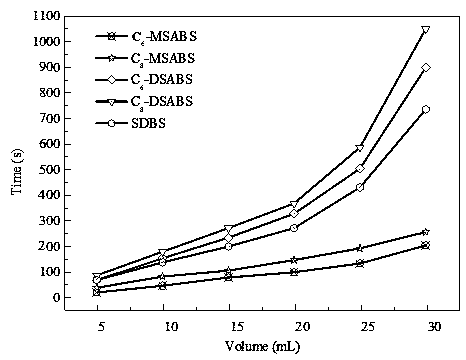
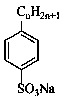
Monoalkyl/dialkyl sodium benzenesulfonate synthesis method
A technology of sodium alkylbenzene sulfonate and synthesis method, which is applied in the field of surfactant synthesis and application, can solve environmental pollution and other problems, and achieve the effects of low equipment requirements, good emulsifying performance and wetting performance, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthesis of a kind of sodium monoalkylbenzene sulfonate in the present embodiment, described synthesis step comprises the following aspects:
[0031] A fixed amount of triethylamine hydrochloride (1.8 mmol), n-decane (5.34 g) and anhydrous aluminum chloride (3.6 mmol) were added to a 100 mL three-necked flask equipped with a reflux condenser, and heated under an argon atmosphere for 60 °C and stirred for 1 h. After the stirring was stopped, the mixture was separated into layers, the upper layer was n-decane, and the lower layer was [Et 3 NH + ][Al 2 Cl 7 - ] Composed of ionic liquid catalysts. According to the molar ratio of benzene:1-hexene being 14:1, benzene was first added to the ionic liquid, and the required 1-hexene was slowly added dropwise under stirring at 60°C. After the alkylation reaction is completed, the mixture is cooled to room temperature and separated into layers. The upper layer is n-decane, alkylbenzene products and unreacted substances, ...
Embodiment 2
[0035] The synthesis of a kind of sodium dialkylbenzene sulfonate in the present embodiment, described synthesis step comprises the following aspects:
[0036] A fixed amount of triethylamine hydrochloride (1.8 mmol), n-decane (5.34 g) and anhydrous aluminum chloride (3.6 mmol) were added to a 100 mL three-necked flask equipped with a reflux condenser, and heated under an argon atmosphere for 60 °C and stirred for 1 h. After the stirring was stopped, the mixture was separated into layers, the upper layer was n-decane, and the lower layer was [Et 3 NH + ][Al 2 Cl 7 - ] Composed of ionic liquid catalysts. According to the molar ratio of benzene:1-hexene being 1:6, benzene was first added to the ionic liquid, and the required 1-hexene was slowly added dropwise under stirring at 60°C. After the alkylation reaction is completed, the mixture is cooled to room temperature and separated into layers. The upper layer is n-decane, alkylbenzene products and unreacted substances, and...
Embodiment 3
[0040] The synthesis of a kind of sodium monoalkylbenzene sulfonate in the present embodiment, described synthesis step comprises the following aspects:
[0041] A fixed amount of triethylamine hydrochloride (1.5 mmol), n-decane (5.34 g) and anhydrous aluminum chloride (3.9 mmol) were added to a 100 mL three-necked flask equipped with a reflux condenser, and heated under an argon atmosphere for 60 °C and stirred for 1 h. After the stirring was stopped, the mixture was separated into layers, the upper layer was n-decane, and the lower layer was [Et 3 NH + ][Al 2 Cl 7 - ] Composed of ionic liquid catalysts. According to the molar ratio of benzene:1-octene being 12:1, benzene was first added to the ionic liquid, and the required 1-octene was slowly added dropwise under stirring at 60°C. After the alkylation reaction is completed, the mixture is cooled to room temperature and separated into layers. The upper layer is n-decane, alkylbenzene products and unreacted substances, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com