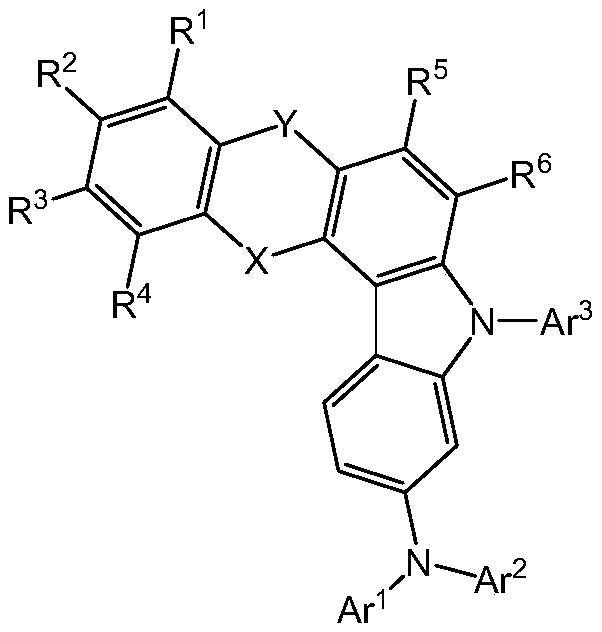
Organic light-emitting material and applications thereof
A luminescent material and organic technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of electron and hole mismatch, shortened life, and efficiency roll-off in the luminescent layer, and achieve good luminous efficiency, Reduced lighting voltage and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
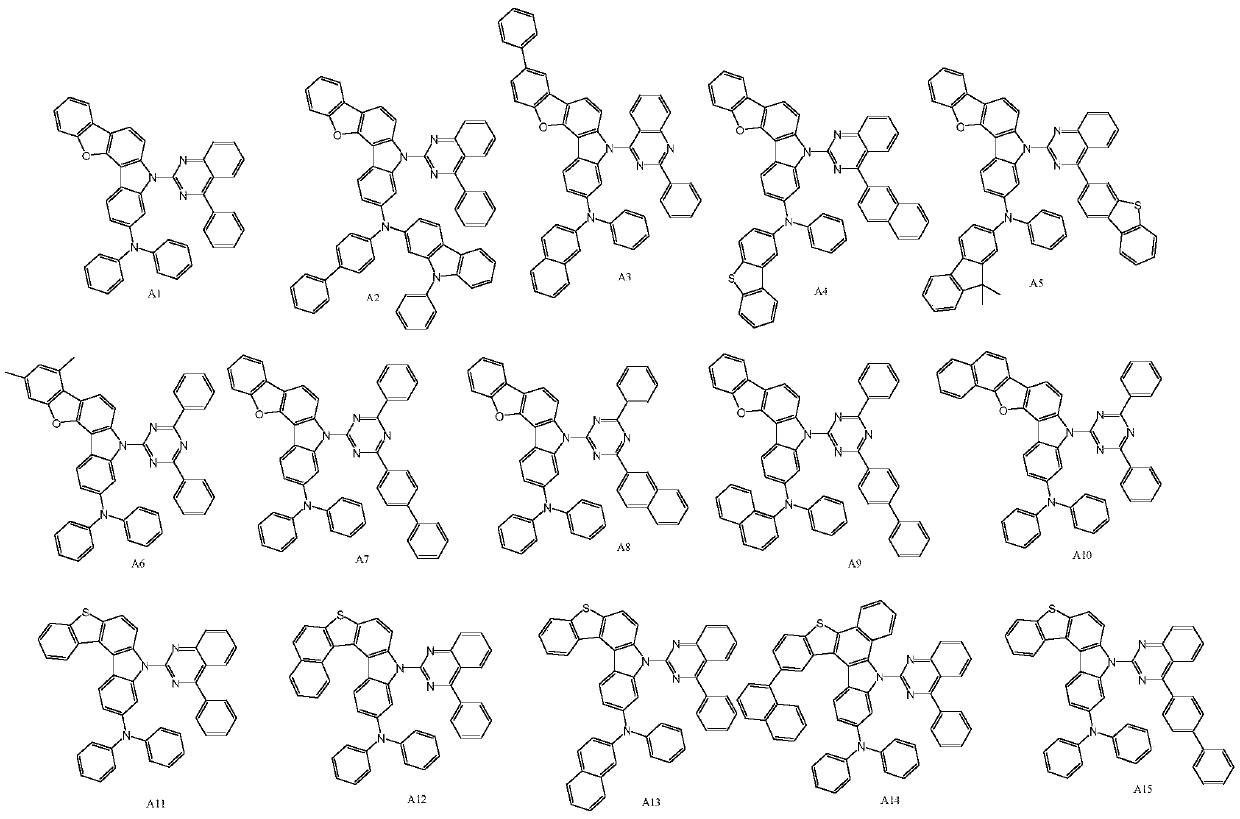
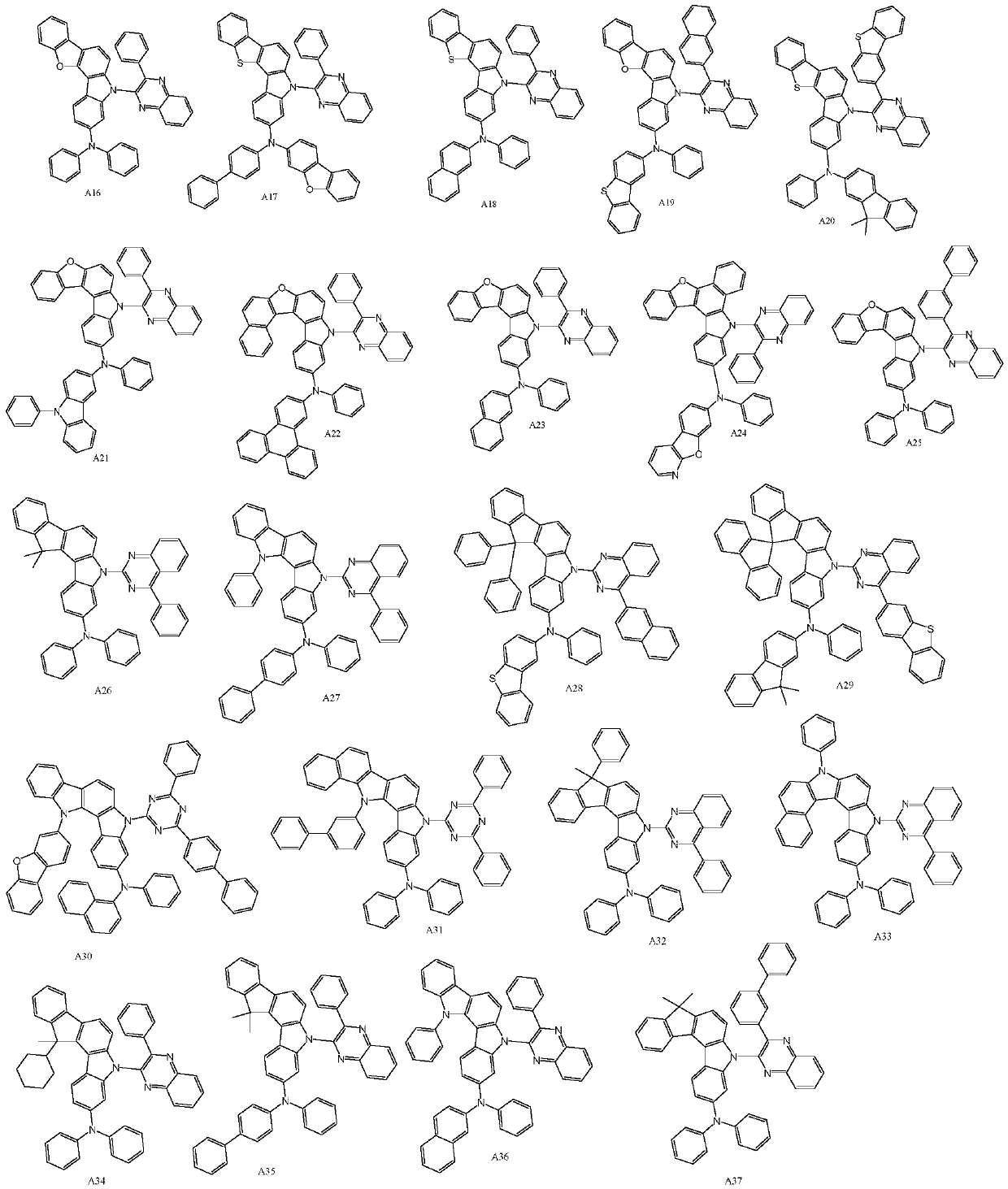
[0033] For the synthesis of compound A1, the reaction equation is as follows:
[0034]
[0035] The synthesis method is as follows:
[0036] (1) Take 21.2g (100mmol) 4-dibenzofuran boronic acid, 32.6g (100mmol) 2-iodo-5-bromonitrobenzene, (1%) Pd(PPh 3 ) 4 , Sodium carbonate 40g (300mmol), toluene (800mL), ethanol (200mL) and water (200mL), heated to reflux, reacted for 8h, the reaction is complete; the reaction solution is extracted with ethyl acetate, the organic phase is concentrated to obtain a yellow solid A;
[0037] (2) Take 100mmol Intermediate A, add 1000mL o-dichlorobenzene solution, add (300mmol) triphenylphosphine, heat to reflux, react for 12h, the reaction is complete; distill off the solvent, silica gel column chromatography, separation, get the middle Body B;
[0038] (3) In the reaction flask, add (100mmol) intermediate B, 16.5g (100mmol) diphenylamine, 0.9g (0.785mmol, 0.5%) Pd 2 (dba) 3 1500mL of toluene and 40g (300mmol) of sodium tert-butoxide, react at 100°C for ...
Embodiment 2
[0042] For the synthesis of compound A5, the reaction equation is as follows:
[0043]
[0044] The synthesis method is as follows:
[0045] (1) Take 21.2g (100mmol) 4-dibenzofuran boronic acid, 32.6g (100mmol) 2-iodo-5-bromonitrobenzene, (1%) Pd(PPh 3 ) 4 , Sodium carbonate 40g (300mmol), toluene (800mL), ethanol (200mL) and water (200mL), heated to reflux, reacted for 8h, the reaction is complete; the reaction solution is extracted with ethyl acetate, the organic phase is concentrated to obtain a yellow solid A;
[0046] (2) Take 100mmol Intermediate A, add 1000mL o-dichlorobenzene solution, add (300mmol) triphenylphosphine, heat to reflux, react for 12h, the reaction is complete; distill off the solvent, silica gel column chromatography, separation, get the middle Body B;
[0047] (3) In the reaction flask, add (100mmol) Intermediate B, 16.5g (100mmol) 2-(9,9-dimethylfluorene)-aniline, 0.9g (0.785mmol, 0.5%) Pd 2 (dba) 3 1500mL of toluene and 40g (300mmol) of sodium tert-butoxide, ...
Embodiment 3
[0051] For the synthesis of compound A13, the reaction equation is as follows:
[0052]
[0053] The synthesis method is as follows:
[0054] (1) Take 21.2g (100mmol) 1-dibenzothiophene boronic acid, 32.6g (100mmol) 2-iodo-5-bromonitrobenzene, (1%) Pd(PPh 3 ) 4 , Sodium carbonate 40g (300mmol), toluene (800mL), ethanol (200mL) and water (200mL), heated to reflux, reacted for 8h, the reaction is complete; the reaction solution is extracted with ethyl acetate, the organic phase is concentrated to obtain a yellow solid A;
[0055] (2) Take 100mmol Intermediate A, add 1000mL o-dichlorobenzene solution, add (300mmol) triphenylphosphine, heat to reflux, react for 12h, the reaction is complete; distill off the solvent, silica gel column chromatography, separation, get the middle Body B;
[0056] (3) In the reaction flask, add (100mmol) Intermediate B, (100mmol) 2-(2-naphthyl)-aniline, 0.9g (0.785mmol, 0.5%) Pd 2 (dba) 3 1500mL of toluene and 40g (300mmol) of sodium tert-butoxide, react at 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com