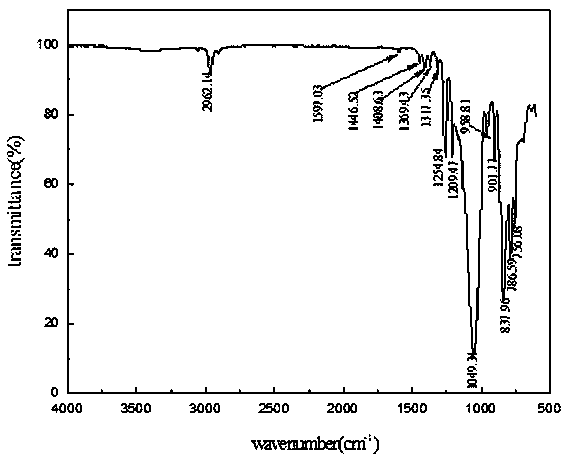
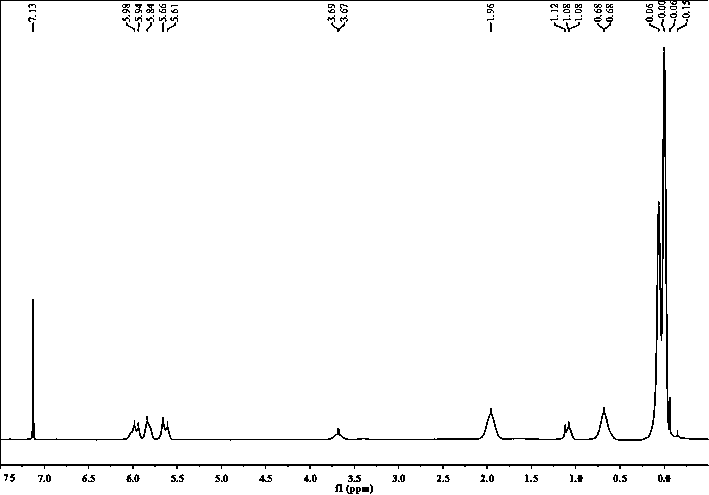
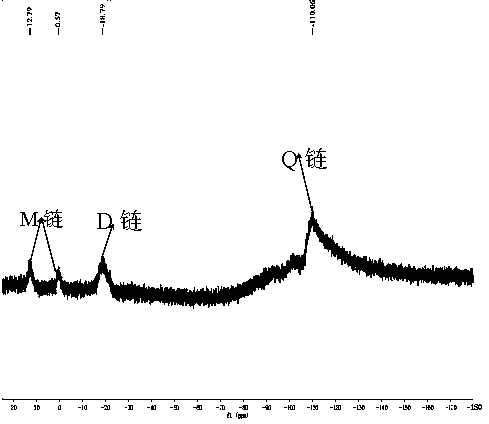
Chemical crosslinking curable fluorine-containing MDQ (mono-di-quarter) type silicone resin and preparation method thereof
A chemical cross-linking, silicone resin technology, applied in the field of organic polymer chemistry, can solve the problems of resin not curing, poor antifouling performance, etc., to avoid gaps or cracks, mild reaction conditions, uniform and stable structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 100mL three-necked flask equipped with a mechanical stirring device, a thermometer, a constant pressure dropping funnel and a condensing device, add 20.6925g (1.1496mol) of deionized water, 7.865g (0.1462mol) of absolute ethanol, 1.3348g (0.0082mol) mol) hexamethyldisiloxane, 1.276g (0.0069mol) 1,3-divinyl-1,1,3,3-tetramethyldisiloxane (referred to as vinyl double head, the same below) and 14.4291g (0.0714mol) of methyltrifluoropropyldimethoxysilane, after starting stirring to make it evenly mixed, 4.72g (0.0465mol) of concentrated hydrochloric acid with a concentration of 36wt% was added dropwise to the above mixture within 1min . After the concentrated hydrochloric acid was added dropwise, the temperature of the kettle liquid was raised from room temperature to 50° C. over 10 minutes, and then 21.3145 g (0.1506 mol) of tetraethoxysilane was added dropwise into the reaction kettle at a constant speed over 20 minutes. After the tetraethoxysilane was added dropwise...
Embodiment 2
[0046] In a 100mL three-necked flask equipped with a mechanical stirring device, a thermometer, a constant pressure dropping funnel and a condensing device, 18.0292g (1.0016mol) of deionized water, 8.1455g (0.1771mol) of absolute ethanol, 3.2298g (0.01994 mol) hexamethyldisiloxane, 1.641g (0.0888mol) 1,3-divinyl-1,1,3,3-tetramethyldisiloxane and 20.355g (0.1008mol) methyltrifluoro Propyldimethoxysilane, after starting stirring to make it evenly mixed, 4.18 g (0.0412 mol) of concentrated hydrochloric acid with a concentration of 36 wt % was added dropwise to the above mixture within 1 min. After the concentrated hydrochloric acid was added dropwise, the temperature of the kettle liquid was raised from room temperature to 50° C. over 5 minutes, and then 20.7757 g (0.0999 mol) of tetraethoxysilane was added dropwise into the reaction kettle at a constant speed over 25 minutes. After the tetraethoxysilane was added dropwise, the temperature of the material in the kettle was raised...
Embodiment 3
[0051] In a 100mL three-neck flask equipped with a mechanical stirring device, a thermometer, a constant pressure dropping funnel and a condensing device, add 23.7051g (1.317mol) of deionized water, 5.2678g (0.1145mol) of absolute ethanol, 3.9409g (0.0243 mol) hexamethyldisiloxane, 4.9687g (0.0267mol) 1,3-divinyl-1,1,3,3-tetramethyldisiloxane and 7.3439g (0.0364mol) methyltrifluoro Propyldimethoxysilane, after starting the stirring to make it evenly mixed, 4.2753g (0.0713mol) of acetic acid was added dropwise to the above mixture within 2min. After the acetic acid was added dropwise, the temperature of the kettle liquid was raised from room temperature to 40° C. over 3 minutes, and then 20.8098 g (0.0999 mol) of tetraethoxysilane was added dropwise into the reaction kettle at a constant speed over 5 minutes. After the tetraethoxysilane was added dropwise, the temperature of the material in the kettle was raised to 70°C and maintained at this temperature for 2 hours, then the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com