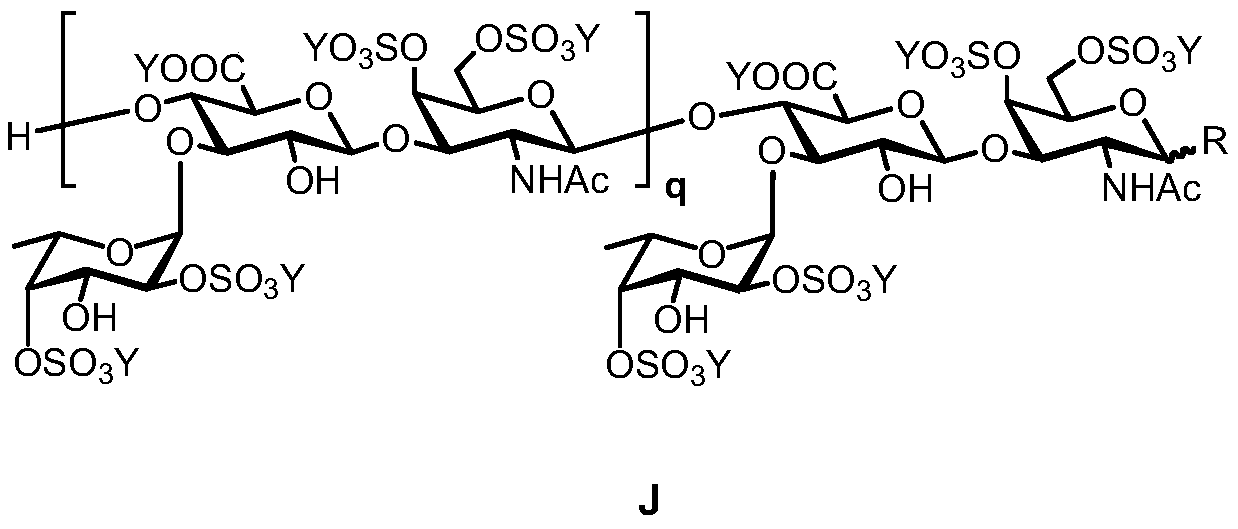
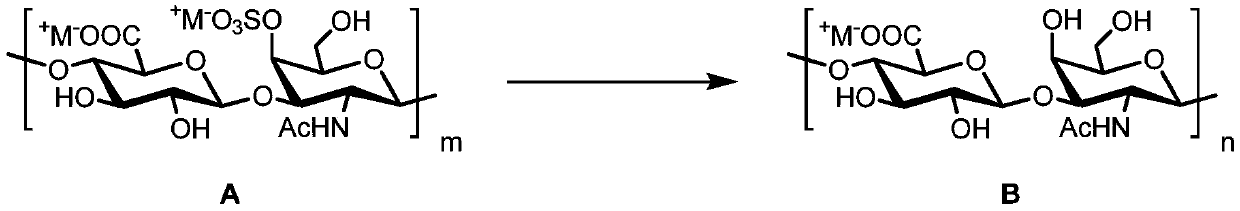
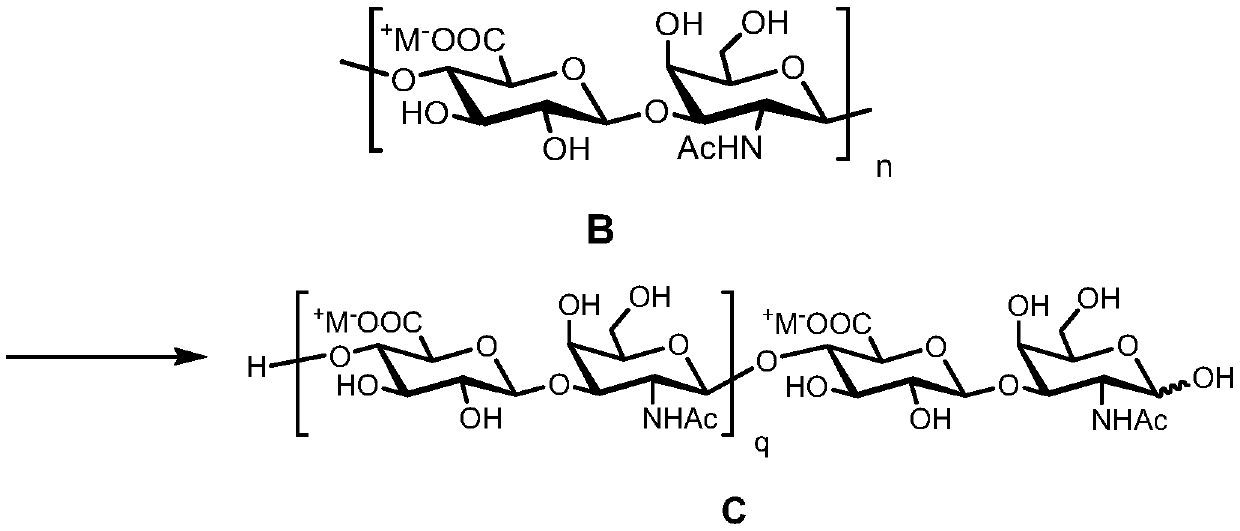
Fucosylation chondroitin sulfate oligosaccharide and preparation method, compound and application thereof
A technology of fucosylation and sulfated cartilage, which is applied in the directions of esterification and saccharide, chemical instruments and methods, and drug combination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Synthesis of Chondroitin Glycans
[0097] Take 25g of commercially available chondroitin sulfate A sodium salt and place it in 2.5L of hydrochloric acid-methanol solution (0.5% acetyl chloride, v / v), stir at room temperature for 24 hours, remove the solvent by suction filtration, and place the obtained solid in 2.5L of the same concentration again hydrochloric acid-methanol solution. This operation was repeated for three times, and the finally obtained solid was dissolved in 750 mL of 0.1 M sodium hydroxide solution, and stirred at room temperature for 24 hours. Add IR-120 cation exchange resin to neutralize the solution to pH = 3.5, remove the resin by filtration, add appropriate amount of sodium hydroxide solid to adjust to pH = 8.0, concentrate until dry to obtain white syrup. Dissolve the syrup with a small amount of distilled water and slowly drop it into 2000 mL of vigorously stirred absolute ethanol to precipitate a white precipitate, collect the prec...
Embodiment 2
[0098] Example 2: (β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D-galactopyranosyl)-(1→4)- (β-D-glucopyranose)-(1→3)-2-deoxy-N-acetylamino-D-galactopyranose disodium salt, (β-D-glucopyranose) -(1→3)-(2-deoxy-N-acetylamino-β-D-galactopyranosyl)-(1→4)-(β-D-glucopyranosyl)-(1→ 3)-(2-Deoxy-N-acetylamino-β-D-galactopyranosyl)-(1→4)-(β-D-glucopyranosyl)-(1→3)-2 -Deoxy-N-acetylamino-D-galactopyranose trisodium salt and (β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D -galactopyranosyl)-(1→4)-(β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D-pyranose Lactosyl)-(1→4)-(β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D-galactopyranosyl)- Synthesis of (1→4)-(β-D-glucopyranose)-(1→3)-2-deoxy-N-acetylamino-D-galactopyranose tetrasodium salt
[0099] Take 15.0 g of the chondroitin polysaccharide obtained in Example 1 and place it in 750 mL of 0.10 M sodium acetate-acetic acid buffer solution containing 0.15 M sodium chloride at pH = 5.00, and use a constant temperature ...
Embodiment 3
[0102] Example 3: Azide-[(β-D-glucopyranosyl)-(1→3)-(2-deoxy-2-N-acetylamino-β-D-galactopyranosyl)-( 1→4)-(β-D-glucopyranosyl)-(1→3)-(2-deoxy-2-N-acetylamino-β-D-galactopyranosyl)] disodium salt synthesis
[0103] Take (β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D-galactopyranosyl)-(1→4)-(β- D-glucopyranose)-(1→3)-2-deoxy-N-acetylamino-D-galactopyranose disodium salt (660mg, 0.81mmol), N-methylmorpholine (2.4mL , 24.0mmol), and sodium azide (3.0g, 46.0mmol) were dissolved in water (15mL), cooled to 0°C, and 2-chloro-1,3-dimethyl imidazoline chloride (1.4g, 8.4mmol ), stirred for 15 minutes, removed the ice bath, and naturally rose to room temperature to continue the reaction for 36 hours. After the reaction was completed, the solvent was concentrated to remove, Sephadex LH-20 was desalted, and freeze-dried to obtain a white solid (531 mg, 78%). R f =0.24 (n-butanol / water / ethanol / acetic acid=1 / 1 / 1 / 0.05); 1 HNMR (600MHz,D 2 O)δ4.75(1H,d,J=9.4Hz),4.54-4.50(3H,m),4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com