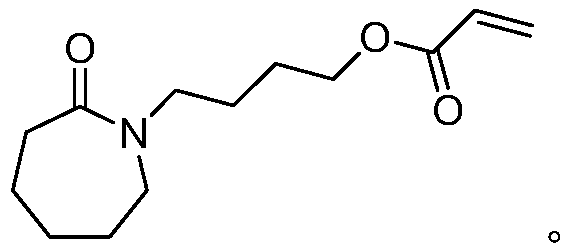
Acrylic ester compound and preparation method and application thereof
A technology of acrylates and epoxy acrylates, which is applied in the field of acrylate compounds and its preparation, can solve problems affecting product purity and product yield, and achieve moderate photocuring time, less skin irritation, and excellent tensile strength Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] (1) Preparation of prepolymer: Add epoxy acrylate, 4-caprolactam butyl acrylate and γ-(methacryloyloxy)propyl trimethoxysilane and initiator diphenyl peroxide in sequence to the polymerization kettle Acyl, stir at 65-70°C for 6-7 hours, and obtain a transparent prepolymer after vacuum degassing;
[0056] (2) Preparation of light-curing coating: 23-30 parts by mass of nano-clay particles dispersed by ultrasound, 3-10 parts by mass of Merck MOK2638 leveling agent, 0.5-2 parts by mass of SN-DEFOAMER 1350 defoamer, 2- 3 parts by mass of triarylsulfonium salt photoinitiator and 60-70 parts by mass of prepolymer are stirred evenly, filled with argon gas and stored in a light-shielded and airtight manner.
[0057] The sixth embodiment of the present disclosure provides a light-curable coating, which is obtained by coating the surface of a substrate with the above-mentioned light-curable coating and curing by ultraviolet light irradiation.
[0058] In one or more examples of t...
Embodiment 1
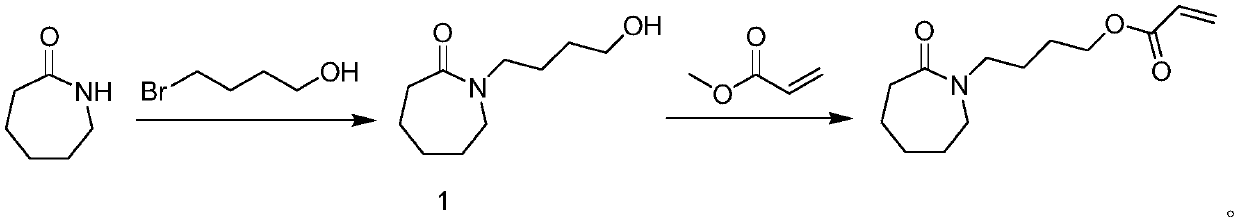
[0061] A method for preparing 4-caprolactam butyl acrylate, specifically comprising the following steps:
[0062] Preparation of organotin catalyst supported on mesoporous silica gel:
[0063] Add 10.0kg of deionized water, 1.0kg of cetyltrimethylammonium bromide, 2.0kg of ethyl orthosilicate, and 0.2kg of dibutyltin dilaurate to the reaction kettle in sequence, stir at 50°C for 1 hour, and then add Ammonia water was gradually added dropwise to adjust the pH value to 8-9, and the reaction was continued for 4 hours at a constant temperature of 50°C. After the reaction stopped, it was spray-dried and granulated, calcined at 350°C for 4 hours, and ground. Then dip in the ethanol solution of 0.6kg dibutyltin maleate and 0.6kg stannous octoate, the ethanol consumption is 2.4kg. After overnight, it was filtered and dried in vacuum at a temperature of 80-90°C to obtain an organotin catalyst supported on mesoporous silica gel, and the calculated loading rate was 30.6%.
[0064] Syn...
Embodiment 2
[0070] A method for preparing 4-caprolactam butyl acrylate, specifically comprising the following steps:
[0071] Add 10.0kg of deionized water, 1.6kg of cetyltrimethylammonium bromide, 2.3kg of ethyl orthosilicate, and 0.25kg of dibutyltin dilaurate to the reaction kettle in turn, stir at 40°C for 2 hours, and then add to the system Ammonia water was gradually added dropwise to adjust the pH value to 8-9, and the reaction was continued at a constant temperature of 50°C for 3h. After the reaction stopped, it was spray-dried and granulated, calcined at 400°C for 3 hours, and ground. Then dip in the ethanol solution of 1.0kg dibutyltin maleate and 0.5kg stannous octoate, the ethanol consumption is 4.5kg. After overnight, it was filtered and dried in vacuum at a temperature of 80-90° C. to obtain an organotin catalyst supported on mesoporous silica gel, and the calculated loading rate was 40.0%.
[0072] Synthesis of 4-caprolactam butyl acrylate:
[0073] (1) In the reactor eq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com