Sustained-release nano-calcium peroxide materials, preparation thereof, and method for removing chlorohydrocarbon and/or benzene series from underground water through sustained-release nano-calcium peroxide materials
A calcium peroxide, nanotechnology, applied in the directions of peroxide/peroxyhydrate/peroxyacid/superoxide/ozone, chemical instruments and methods, oxidized water/sewage treatment, etc., to achieve good dispersibility and Mobility, size uniformity, and the effect of improving repair efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) At room temperature, accurately weigh 6g of commercially available anhydrous calcium chloride, add 60mL deionized water to a 500mL beaker, then measure 200mL polyethylene glycol (PEG200) solution, and finally add 30mL to a concentration of 1mol / L ammonia solution, after stirring evenly, place the mixed solution on a magnetic stirrer and stir at a constant speed of 600r / min. The solution contains a certain amount of ammonia, which can generate NH with HCl 4 Cl, promote CaO 2 The reaction formula is as follows:
[0048] 2HCl+2NH 3 →2NH 4 Cl;
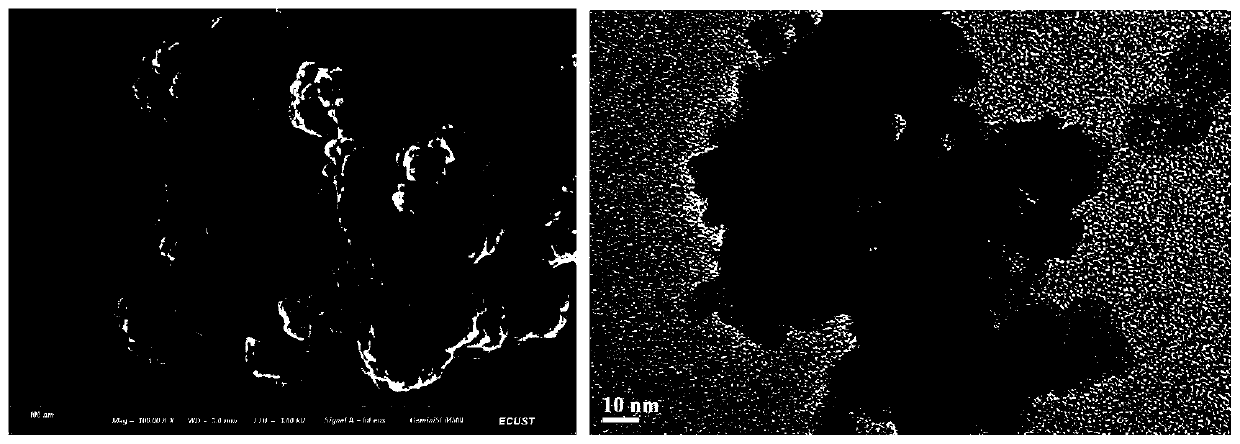
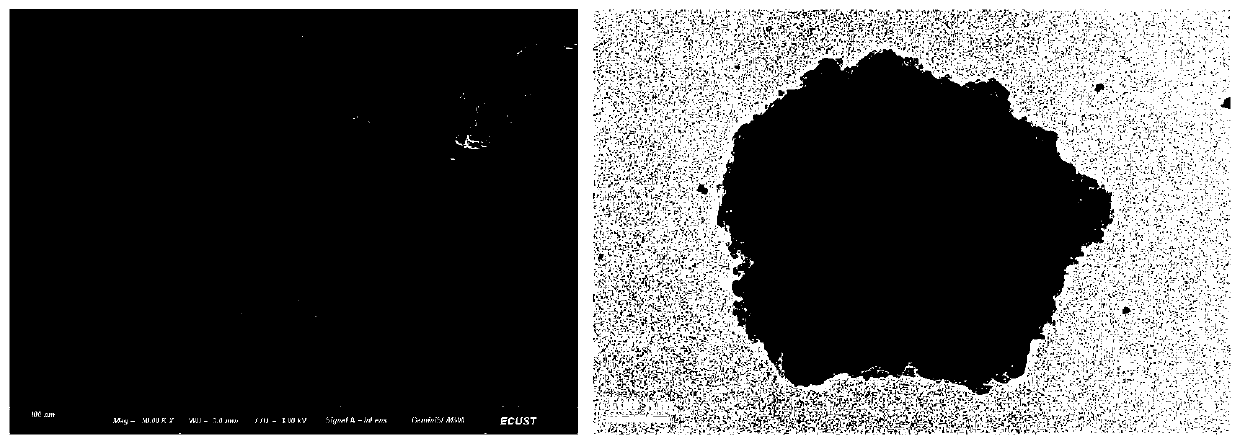
[0049] (2) After the above solution is evenly stirred, add 25 mL of 30% H with a separating funnel at a rate of 3 s / drop 2 O 2 Aqueous solution into the system; with H 2 O 2 With the addition of the solution, the solution gradually changes from colorless to light yellow viscous liquid, which is nano-CaO 2 The slurry was then washed three times with absolute ethanol to remove impurities, and vacuum dried at 60°C for 8 hours to obtain...
Embodiment 2
[0053] (1) At room temperature, accurately weigh 6g of commercially available anhydrous calcium chloride, add 60mL of deionized water to a 500mL beaker, then measure 200mL of polyvinyl alcohol (PVA) solution, and finally add 30mL to a concentration of 1mol / After stirring the L ammonia solution evenly, place the mixed solution on a magnetic stirrer and stir at a constant speed of 600r / min. The solution contains a certain amount of ammonia, which can generate NH with HCl 4 Cl, promote CaO 2 The reaction formula is as follows:
[0054] 2HCl+2NH 3 →2NH 4 Cl;
[0055] (2) After the above solution is evenly stirred, add 25 mL of 30% H with a separating funnel at a rate of 3 s / drop 2 O 2 Aqueous solution into the system; with H 2 O 2 With the addition of the solution, the solution gradually changes from colorless to light yellow viscous liquid, which is nano-CaO 2 The slurry was then washed three times with absolute ethanol to remove impurities, and vacuum dried at 60°C for 8 hours to ob...
Embodiment 3
[0059] (1) At room temperature, accurately weigh 6g of commercially available anhydrous calcium chloride, add 60mL of deionized water to a 500mL beaker, then measure 200mL of diethylene glycol monoethyl ether (DEGMME) solution, and finally add 30mL concentration It is a 1mol / L ammonia solution. After stirring, the mixed solution is placed on a magnetic stirrer and stirred at a constant speed of 600r / min. The solution contains a certain amount of ammonia, which can generate NH with HCl 4 Cl, promote CaO 2 The reaction formula is as follows:
[0060] 2HCl+2NH 3 →2NH 4 Cl;
[0061] (2) After the above solution is evenly stirred, add 25 mL of 30% H with a separating funnel at a rate of 3 s / drop 2 O 2 Aqueous solution into the system; with H 2 O 2 With the addition of the solution, the solution gradually changes from colorless to light yellow viscous liquid, which is nano-CaO 2 The slurry was then washed three times with absolute ethanol to remove impurities, and vacuum dried at 60°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com