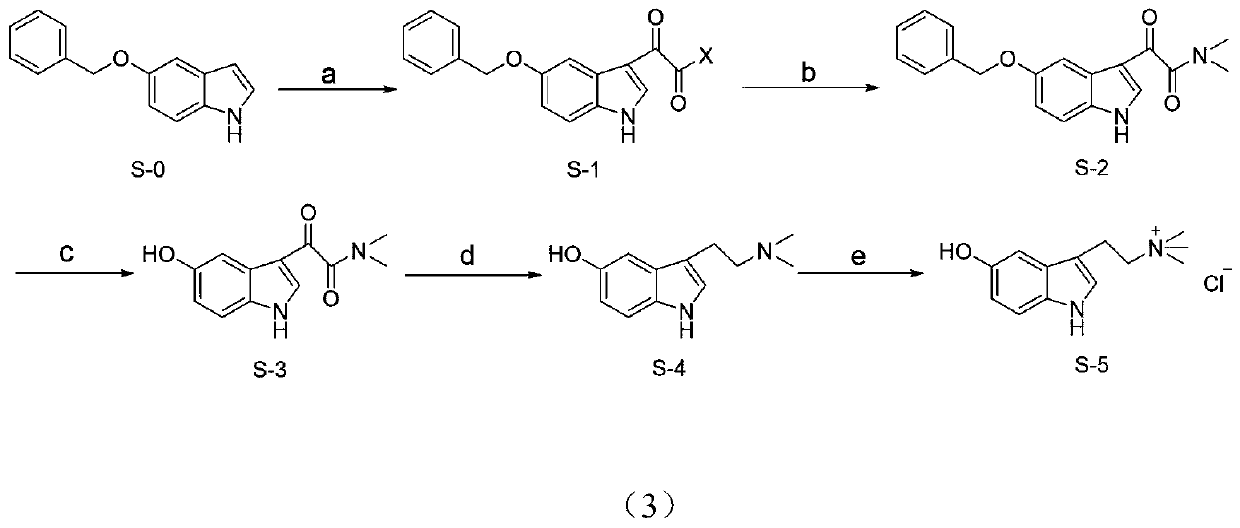
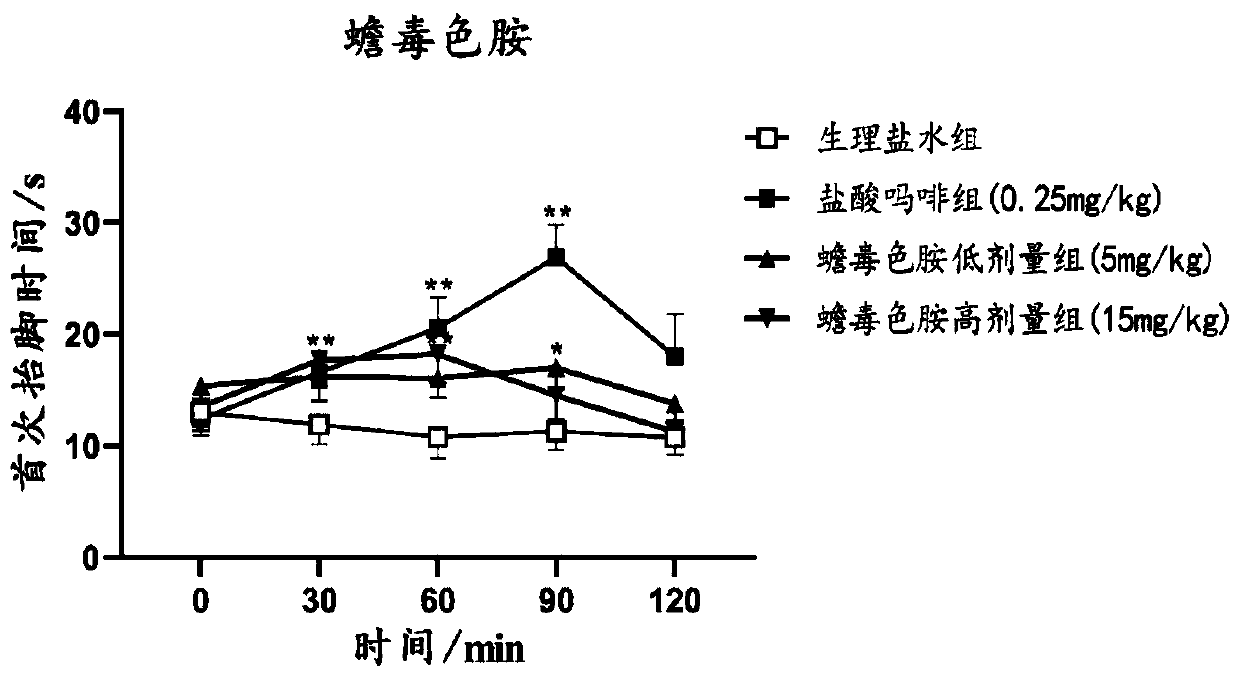
Synthesis method of bufotenin and quaternary ammonium salt thereof and application in preparation of analgesic and anti-inflammatory drugs
A technology of bufotryptamine and its synthesis method, which is applied in the application fields of analgesia, synthesis of bufotryptamine and its quaternary ammonium salt, and anti-inflammatory drugs, and can solve the problem of unsuitable industrial production, highly toxic reagents, and unsuitable In line with the concept of green chemistry and other issues, to achieve the effect of easy purification and separation, low cost and high purity of synthetic products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride (S-1)
[0043] At room temperature, take a 100mL round-bottomed flask, add 2.7mL of anhydrous diethyl ether, oxalyl chloride (175μL, 2.0mmol) in sequence, and stir well, then add 5-benzyloxyindole (220mg, 1.0mmol) into the flask , after fully stirring for 30 minutes, the reaction solution was suction-filtered, and the filter cake was washed repeatedly with ether to obtain about 302.9 mg of a reddish-brown solid precipitate, which was the crude product of S-1, with a yield of 95%.
[0044]
[0045] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide (S-2)
[0046] At room temperature, take a 100mL round bottom flask, add potassium hydroxide (360mg, 6.38mmol) and 4.15mL water and dimethylamine hydrochloride (350mg, 4.25mmol) in turn, mix well, take S-1 (310mg , 1.0mmol) was slowly added to the mixed solution, stirred for 40 minutes, suction filtered, washed with anhydrous ether ...
Embodiment 2
[0062] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride (S-1)
[0063]At room temperature, take a 100mL round-bottomed flask, add 2.7mL of anhydrous diethyl ether, oxalyl chloride (175μL, 2.0mmol) in sequence, and stir well, then add 5-benzyloxyindole (220mg, 1.0mmol) into the flask , after fully stirring for 30 minutes, the reaction solution was suction-filtered, and the filter cake was washed repeatedly with ether to obtain about 299.8 mg of a reddish-brown solid precipitate, which was the crude product of S-1, with a yield of 94%.
[0064]
[0065] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide (S-2)
[0066] At room temperature, take a 100mL round-bottomed flask, add sodium hydroxide (170mg, 4.25mmol) and 4.15mL water and dimethylamine hydrochloride (350mg, 4.25mmol) in turn, mix well, take S-1 (310mg , 1.0mmol) was slowly added to the mixed solution, stirred for 40 minutes, suction filtered, washed with anhydrous ether to...
Embodiment 3
[0082] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-2-oxoacetyl chloride (S-1)
[0083] At room temperature, take a 100mL round-bottomed flask, add 2.7mL of anhydrous diethyl ether, oxalyl chloride (175μL, 2.0mmol) in sequence, and stir well, then add 5-benzyloxyindole (220mg, 1.0mmol) into the flask , after fully stirring for 40 minutes, the reaction solution was suction-filtered, and the filter cake was washed repeatedly with ether to obtain about 293.1 mg of a reddish-brown solid precipitate, which was the crude product of S-1, with a yield of 95%.
[0084]
[0085] Preparation of 2-(5-(benzyloxy)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide (S-2)
[0086] At room temperature, take a 100mL round-bottomed flask, add sodium hydroxide (255mg, 6.38mmol) and 4.15mL water and dimethylamine hydrochloride (350mg, 4.25mmol) in turn, mix well, take S-1 (310mg , 1.0mmol) was slowly added to the mixed solution, stirred for 40 minutes, suction filtered, washed with anhydrous ether t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com